Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
4185
LocalitiesLookout Pass Thallium prospect, Little Valley, Lookout Pass Mining District, Tooele County, Utah, USA
17th Nov 2015 19:03 UTCReiner Mielke Expert

18th Nov 2015 05:34 UTCD Mike Reinke
18th Nov 2015 23:07 UTCNorman King 🌟 Expert
Except for the graphite, this was all added recently.
19th Nov 2015 01:26 UTCReiner Mielke Expert
I am not disputing marcasite, however you did say they looked hexagonal which suggests a pseudomorph of pyrrhotite. That does not mean that there is an pyrrhotite currently there just that at one time there was and it all now replaced by marcasite. This is a common feature here: http://www.mindat.org/photo-392019.html

19th Nov 2015 02:03 UTCAlfredo Petrov Manager
19th Nov 2015 05:23 UTCNorman King 🌟 Expert
I then went back and found these crystals still in matrix (next photo below), but they are hard to find in the dark chert, and they are uncommon. Tiny hexagonal crystals are found here and there, usually in clusters, but very few are still graphitic. I noted that those in my photo have a slight brownish tint, and that bothered me a bit. I am also concerned about the tested material being first embedded in epoxy. But epoxies are rich in oxygen, which is about 3-4 times the content of carbon and should register even better than the carbon, at least more accurately. Tested epoxies do not contain titanium, but it is common in other Lookout Pass samples in addition to the graphite sample. Conversely, other Lookout Pass samples I tested in epoxy did not register any carbon. Therefore, I concluded this analysis is valid.
I would expect graphite to be unstable at high pressures, having a density of roughly 2.1-2.3. In a reducing environment, under pressures existing perhaps a few thousand meters under ground, pyrite or marcasite (or pyrrhotite?) should be more stable phases. (But why bring pyrrhotite into it?-- The hexagonal form would be inherited from graphite.) Near-surface oxidation would tend to promote formation of hematite or goethite. I have yet to post photos of the hexagonal crystals of hematite psd. after marcasite, as I am waiting for more analytical data on them.
These are the reasons why I arrived at my conclusion. Are there thermodynamic or chemical reasons that this is unlikely?
19th Nov 2015 14:16 UTCReiner Mielke Expert
19th Nov 2015 15:21 UTCNorman King 🌟 Expert
I have been in contact with John, and will ask him to review this conversation and for any suggestions he may have.
21st Nov 2015 19:59 UTCNorman King 🌟 Expert
"Carbonization . . . is a part of the metallogenic process, in which organic materials together with gold are remobilized, transported and reprecipitated in the hydrothermal, ore-forming system. Graphite and pitchblende usually are the products of carbonization in Chinese deposits. Carbonization alteration has been reported in the Dongbeizhai, Laerma, and Jinyia gold deposits (Liu, D.S. and others, 1994). Decarbonatization results from oxidation and could decrease the carbon content of the host rock, and promote leaching of gold from its source bed. It is considered to be favorable for gold mineralization (Wang, J. and Du, L.T., 1993). Carbon is mobilized and driven outward by hydrothermal fluids, or in the case of the Betze deposit, Nevada, by contact metamorphism (Leonardson and Rahn, 1996)."
This study shows that carbonization in jasperoids occurs by hydrothermal processes, and that oxidation (very near surface) drives many decarbonization events. Marcasite is typically a low temperature product, and may indeed be more stable under near-surface conditions. Note this passage from the Mindat marcasite page (under “Geological Setting”): "Most frequently found in sedimentary rocks and coal beds, as a replacement mineral forming fossils, it is a mineral of low-temperature, near-surface, environments, forming from acid solutions."
Also, Reiner wrote: "marcasite may be filling a hexagonal void left by graphite." Wouldn't that be a pseudomorph? (Definition of pseudomorph from Mindat glossary: "A mineral sample with the external crystal form of one mineral and the internal chemistry of another; e.g., cubes of geothite after pyrite resulting from oxidation of the ferrous sulfide to ferric oxyhydroxide.")

21st Nov 2015 20:16 UTCAlfredo Petrov Manager
"oxidation (very near surface) drives many decarbonization events"
To play Devil's advocate here: Wouldn't all this carbon-removing oxidation also destroy any marcasite? Marcasite is one of the most sensitive minerals to oxidation. So in order to get a pseudomorph of marcasite after graphite you'd need initial anaerobic conditions, followed by oxidizing conditions to remove the carbon, then back to reducing conditions to deposit the marcasite. Possible, I suppose, but Occam's razor makes it easier for me to believe the pyrrhotite hypothesis.
22nd Nov 2015 01:01 UTCNorman King 🌟 Expert
We can certainly come up with one or more sensible explanations for all of the data. We know that graphite is present in those tiny tabular hexagonal crystals. Marcasite and hematite are both present in the same tiny tabular hexagonal crystals. We don't need to add pyrrhotite to the mix--pyrrhotite for which we have no real evidence, but only the knowledge that pyrrhotite is often replaced by marcasite. I will "not multiply entities needlessly." That was what Occam said we should not do (those were his words, in quotes).
Sorry to spring the hematite on you at this late date. I do have completed analyses for the metallic hematite. Now I fear that the red-orange tabular hexagonal crystals that I have uploaded and labeled cinnabar may actually be hematite. I'll need to get confirmation of that before going to press, as I'm not 100% sure they are the same based upon what I have seen so far.
22nd Nov 2015 01:09 UTCReiner Mielke Expert
22nd Nov 2015 02:22 UTCNorman King 🌟 Expert
20th Dec 2015 16:36 UTCNorman King 🌟 Expert
Relative to earlier comments in this (current) thread by Reiner and Alfredo:
A. J. Hall (1986) showed that pyrite forms in sedimentary settings by bacteriogenic reduction of sea-water sulfate during diagenesis. The iron is derived from iron-bearing detrital sedimentary materials (oxides and silicates). Sulfur-deficient iron sulfides such as pyrrhotite are unstable in even the most reduced sedimentary rocks, so pyrrhotite is not normally present in sedimentary rocks. However, pyrrhotite may form by reduction of previously-existing pyrite or marcasite. In the latter situation, pre-existence of pyrite and/or marcasite can be recognized by relict textures and habits. Note: this is the reverse of what we have been talking about–rather than signs of pre-existing pyrrhotite revealed in pyrite/marcasite occurrences in Lookout Pass cherts, signs of pre-existing pyrite/marcasite would be visible in any pyrrhotite. Such indications are not present because no pyrrhotite has been identified in Lookout Pass samples. As stated above, pyrrhotite is not likely present in Lookout Pass cherts, but I am open to any mechanism that can be elucidated that would allow for it.
Comments by David Von Bargen referenced below were in another thread in which I mentioned Lookout Pass marcasite/pyrite. These comments should be added to this thread (or some other thread relative to Lookout Pass), so I reproduced them below. Otherwise, they most likely would have been lost in an irrelevant context. See http://www.mindat.org/forum.php?read,11,368114,368114#msg-368114.
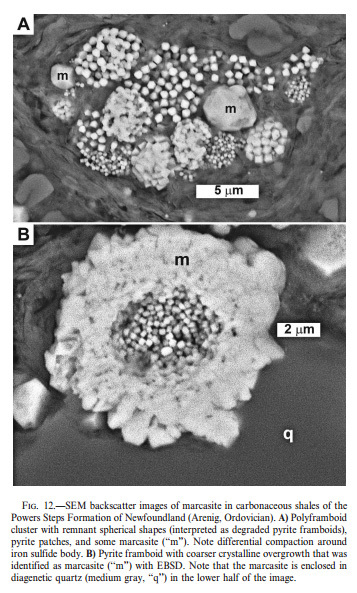
Hall (1986) did not attempt to distinguish between disulfides (pyrite and marcasite), noting that these minerals generally have a similar distribution. Schieber (2011) showed numerous examples of intimately intermixed pyrite and marcasite in black shales, identified by textures and habits using EBSM micrography (note figure from that paper below; several other photos of pyrite/marcasite in dark shales were also presented in that paper). Pyrite formed first, then marcasite. Of course, intergrowth of those mineral phases may frustrate most any analytical attempt to determine which one is present. Actually, the question, “Which ONE is present?” would be the wrong question, since both are likely present in intimate association. Perhaps BDSM would work in the Lookout Pass situation also. I brought it up (see excerpt from other thread below), but let me reiterate that just because it is possible, in principle, to make those determinations does not mean it makes much sense to expend the time, energy, and expense to do so.
References (available on-line):
Hall, A. J. (1986), Pyrite-pyrrhotine redox reactions in nature: Min. Mag., v. 50, p. 223-229.
Schrieber, J. (2011), Marcasite in black shales—a mineral proxy for oxygenated bottom waters and intermittent oxidation of carbonaceous muds: J. Sed. Res., v. 81, p. 447-458.
* * * * * * *
Earlier thread not related to Lookout Pass: http://www.mindat.org/forum.php?read,11,368114,368114#msg-368114
relevant post by Norman King
Re: Types of specimen analysis and the costs.
December 05, 2015 01:49PM
I will add that people who need (or think they need) a "definite ID" are bound for disappointment. However, some of them may not be happy until they get a "definite ID," especially if they paid for an analysis, so someone may say, "Yes this is definite" just to make him/her happy and maybe get more business. But neither analytical data nor lists of physical properties will always require a single INTERPRETATION. Consider, as examples, the partially erroneous list of physical properties (cleavage and luster) published for parapierrotite, and just try getting an XRD for isolated iron disulfide crystals averaging 0.04 mm embedded in chert (since EDS won't distinguish pyrite from marcasite). I have concluded that I will never be able to determine what any given speck or other tiny spot is in a sample of chert from Lookout Pass without analysis, and there may be millions of such specks, and larger crystals can be divided up into smaller segments endlessly. Shall we analyze all of them? Welcome to the real world.
posted by David Von Bargen in response
Re: Types of specimen analysis and the costs.
December 05, 2015 02:37PM
"and just try getting an XRD for isolated iron disulfide crystals averaging 0.04 mm embedded in chert (since EDS won't distinguish pyrite from marcasite)." - You need to use the right tools. A polished section of the material will allow you to examine many examples in a specimen. Optically there is a very high anisotrophy for marcasite while pyrite is isotropic or displays very weak anisotrophy.
Sometimes the older methods are a whole lot better than all these new fangled techniques. Don Peck's book is invaluable resource for mineral ID using older techniques.
20th Dec 2015 16:52 UTCReiner Mielke Expert
I will be looking forward to the results of John's work on your material. It would really be something if it turned out they are pseudos of graphite. You sure have taken our understanding of that deposit and the mineralogy of the Tl minerals a long way, thanks for all your hard work.

13th Sep 2017 07:53 UTCPrakash Chandra
Sir, I want to know how to differentiate between Marcasite and Pyrite in optical microscope ?
Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 20, 2024 04:16:41
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 20, 2024 04:16:41


















Lookout Pass Thallium prospect, Little Valley, Lookout Pass Mining District, Tooele County, Utah, USA