Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Fakes & FraudsFake Trinitite
21st Aug 2009 16:19 UTCEugene & Sharon Cisneros Expert
Editorial by Eugene Cisneros
It's a sad commentary, but true. Some unethical people have been selling fake Trinitite on the web and online auctions. We were recently offered some of this material as well. If it's too green, too translucent or too inexpensive to be true, it probably isn't real Trinitite! So how does the collector know if a specimen is authentic? Well, usually the only means for the collector to eliminate the fake stuff is to use a sensitive dosimeter and take a reading to verify that residual induced radiation exists. This will be very small, but it can be detected. This will not rule out the possibility that clever fakers will not add some small amount of weakly radioactive material to their formula. The only way to be 100% certain of authenticity is by means of nuclear energy spectroscopy. I have worked under contract of the Department of Energy, at a national laboratory, for the past 37 years and have the resources to perform these tests. Thus, we at Mineralogical Research Company can assure you of the authenticity of all of the Trinitie specimens that we make available to you - - - we guarantee our specimens 100%.
The energy spectra, below, shows the radioactive isotope Cesium 137 photopeak at ~666 keV. This radionuclide has a half life of 30.2 years and is one of several unique byproducts of nuclear fission or atomic detonations. It is present in all authentic Trinitite specimens.
21st Aug 2009 17:18 UTCJustin Zzyzx Expert
Do you have a picture of any fake pieces? I took a whole bunch of REAL trinitite and put it into some muratic acid and the end result was some very pretty brighter green material. I would love to see some pictures of what people are trying to pass off as fake...

21st Aug 2009 20:37 UTCLachrisha Smith
22nd Aug 2009 02:55 UTCJustin Zzyzx Expert
22nd Aug 2009 18:24 UTCJustin Zzyzx Expert
=)
22nd Aug 2009 18:43 UTCDon Saathoff Expert
Don S.
22nd Aug 2009 22:11 UTCPaul Brandes 🌟 Manager
I remember the last time I was out at the Trinity Site, there wasn't a lot left laying on the ground, but I did manage to find a few little pieces here and there. If you get the chance to go out there (first Saturday in April and October), there is about a 6 ft by 12 ft covered area with glass that is an untampered patch of trinitite that allows people to see what the ground looked like after the blast. The last time I stopped at the Blanchard Rock Shop, Allison still had a fair amount of "real" trinitite for sale. I thought the prices were fair considering no more is being made. If your in the Socorro area, you may want to drive out west to Magdalena and stop at Bill's Rock Shop. It too had trinitite the last time I stopped in and talked with Helen Dobson. I believe the mineral museum on the New Mexico Tech campus also has trinitite from time to time as well.
23rd Aug 2009 04:02 UTCEugene & Sharon Cisneros Expert
No pics, so I guess it didn't happen. It didn't happen that I was ignorant enough to buy it, that is. ;)
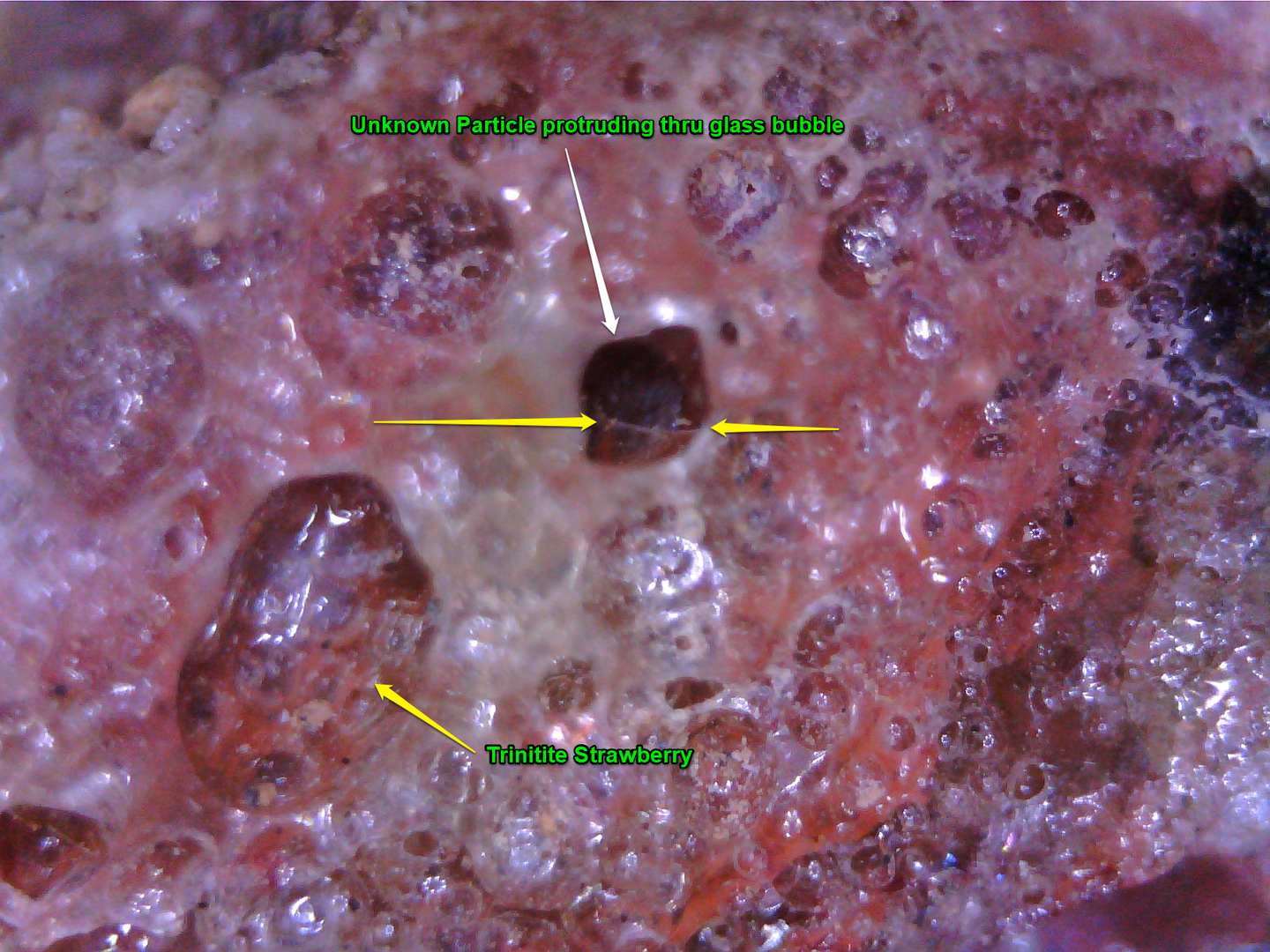
Attached are some pic of real Trinitite for anyone who is interested.
Gene
23rd Aug 2009 04:05 UTCSteve Hardinger 🌟 Expert
23rd Aug 2009 18:10 UTCRichard Dale Expert
24th Aug 2009 03:41 UTCGail Spann Manager
We went up to the Blanchard Mine and spent a great day finding fluorite, galena, linarite and drusy quartz.
To the originator of this post, thank you for your warnings!
2nd Sep 2009 03:33 UTCDr. Paul Bordovsky
tired of the green chile cheeseburgers in San Antonio, NM.
2nd Sep 2009 20:10 UTCDon Saathoff Expert
7th Sep 2009 01:29 UTCGail Spann Manager
I am sorry to hear that Ray DeMark's wife, Judy, just passed away. Ray is one of the owners of the Bingham mine.
Judy was a very outspoken, delightful character and I will miss her greatly.

15th Feb 2011 02:12 UTCJMckethen
15th Feb 2011 03:02 UTCJim Bean 🌟

15th Feb 2011 04:20 UTCDarren Court
Darren
p.s. - have never seen black, and you have to be careful, as there is stuff floating around out there from the Nevada test site, as well as various missile tests, that is claimed to be trinitite. Post your photo, would love to see it!

23rd May 2012 16:57 UTCJessS
I had it in a little plastic bag on a high shelf in my apartment. Secured well or so I thought. I have a cat that likes to steal small plastic bags. This morning my trinitite is missing. I searched for an hour but fear that kitty hid it from me. That cat likes to chew plastic bags. I am very concerned - apart from hunting the apartment with the Ludlum detector does anyone have any suggestions? Especially for how to treat my cat.
23rd May 2012 17:06 UTCDan Fountain
-------------------------------------------------------
> ...does anyone
> have any suggestions? Especially for how to treat
> my cat....
Please don't feed the trolls! ;-)
23rd May 2012 17:18 UTCJolyon Ralph Founder

23rd May 2012 17:55 UTCJessS
23rd May 2012 18:01 UTCJolyon Ralph Founder
No. Yes. No.
23rd May 2012 19:09 UTCDon Saathoff Expert
Don
23rd May 2012 19:25 UTCRob Woodside 🌟 Manager

23rd May 2012 19:28 UTCJessS

23rd May 2012 19:28 UTCAli

23rd May 2012 20:34 UTCJessS
23rd May 2012 22:08 UTCEugene & Sharon Cisneros Expert
I wouldn't be too concerned about the cat.
1. The cat most likely would not eat a piece of Trinitite.
2. Even if the cat did eat it, it would pass through in a day and there would not be any induced radiation. If it didn't pass, you would have a constipated cat by now.
3. If the cat ingested some of the dust into its lungs, there isn't much to be done. The cat will most likely be long gone from natural causes before it gets lung cancer.
4. I have handled thousands, yes thousands, of pieces of Trinitite and none have had rad levels greater than 0.5mr/hr and most are far less than that. Since you say that you measured 3mr/hr, I would be suspicious of the origin of the material.
Good luck to the cat and I hope that you find your specimen of what ever it is.
Cheers,
Gene

25th May 2012 22:12 UTCJeff A
I am definitely not a rock hound, I am more interested in the Manhattan Project and thought some trinitite would be nice to have. I just bought a small piece from Allison at Blanchard Rock Shop in May 2012.
Very happy.
26th May 2012 03:32 UTCEugene & Sharon Cisneros Expert
If you are interested in the Manhattan Project you might like these books.
The Day the Sun Rose Twice - The story of the Trinitiy Site Nuclear Explosion
Prof. Ferenc Szasz
The Los Alamos Primer - First lectures on How to Build an Atomic Bomb
Robert Serber
Gene

11th Sep 2012 23:35 UTCWM Kolb

12th Sep 2012 14:27 UTCDarren Court
Darren
12th Sep 2012 16:44 UTCEugene & Sharon Cisneros Expert
Thanks for the clarification of the blue color. We have, on occasion, found glassy "robin’s egg blue" streaks in a few of our Trinitite specimens. More commonly, we find red (copper), black (?) and white (probably fused feldspar) as we sort specimens. A solid piece of blue is something that we have not seen in the handling of thousands of specimens. If you could post a picture of the specimen, it would certianly be appreciated.
Gene

20th Mar 2014 21:57 UTCsam L
-thanks
20th Mar 2014 22:34 UTCPaul Brandes 🌟 Manager

21st Mar 2014 01:17 UTCMichael Hatskel

16th Apr 2014 05:46 UTCMe
These were given to me from a family members fairly extensive rock & mineral collection after they were deceased. These people lived within 100 miles of Trinity Site, White Sands Missile Range. I do not have spectra or measurements with a dosimeter for these. I will never sell them, but I am almost completely certain that these are from Trinity Site, 7/16/45. For your reference.
JG

28th Feb 2015 18:54 UTCTheKnight
-Matt
28th Feb 2015 22:11 UTCDon Saathoff Expert
Your trinitite looks identical to the trinitite in our collection which I know is real - self collected by repeatedly tying my shoe!! Radioactivity is slight...
Don S.

11th Jun 2017 22:13 UTCScott Trez
Hopefully someone will chime in about this question.
You hear talk about "fake" trinitite...so far just talk from what I see.
My question is, does anybody have any Fake Trinitite that they can post a pic or two of such?
I find it odd that no pictures can be found of such.
Thanks in advance,
Scott

10th Sep 2017 18:19 UTCLeeS. (Lee Sandquist)
10th Sep 2017 21:49 UTCPaul Brandes 🌟 Manager
Photos would be very helpful.
If in doubt, I would take it to either the geology dept. at University of New Mexico (if you're near Albuquerque) or to the Mineral Museum at New Mexico Tech in Socorro.

21st Sep 2017 01:06 UTCScott Trez
I have acquired a very beautiful collection of Trinitite and look for unique pieces to photo and share...it truly is a remarkable man-made material with extreme historical value.









Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 16, 2024 19:05:00
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 16, 2024 19:05:00