Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
GeneralCorundum

10th Mar 2015 09:25 UTCAnonymous User
I hope someone here can enlighten me.
Thank you.
10th Mar 2015 11:37 UTCJosé Zendrera 🌟 Manager
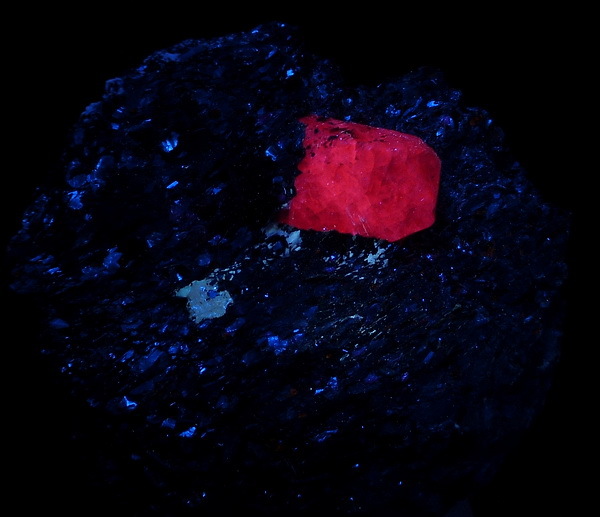
Some corundum specimens have both red (chromium) and blue (iron) chromophores giving a violet/purple color, that is what could happen here. In these cases ruby fluorescence can prevail when iron content is low.
Synthetic corundum usually do not have solid inclusions, maybe you can check this with a loupe or microscope.
10th Mar 2015 12:50 UTCOwen Melfyn Lewis
Strong fluorescence and the presence of an appreciable amount of Fe are never found together. Fluorescence in mined ruby can vary strong to inert and still be ruby by colour (rather dark).
Neither crystal form nor the absence of inclusions is proof that a specimen is not of synthetic origin only it has probably not made by the flame fusion process.
A picture of the specimen would be helpful. You have not even said whether it has a crystal form, is broken rough or of a cut and polished stone. So, for the moment, there is no sensible advice that can be given - except look closer; test and report more.
One tip. If the stone has been faceted and polished, be sure you photograph it face down on a diffused light source (small torch covered by a white handkerchief will do in an emergency). The camera needs to be vertical relative to the plane on which the stone is resting. This is not a glamorous view but if the image is SHARP will show details that cannot be seen in other views.

10th Mar 2015 23:58 UTCAnonymous User

14th Mar 2015 07:43 UTCAnonymous User
Here's the photo's, please check it out.
14th Mar 2015 16:50 UTCOwen Melfyn Lewis
The blue crystal shows, on the top face - a perfect cleavage? - a pronounced pattern of parallel lines intersecting at close to 90 deg. So not corundum, I think. Kyanite might be a possibility? Are you able to check the pleochroism?

14th Mar 2015 18:50 UTCDoug Daniels
The patterns on the blue crystal, certainly not like any I've seen on corundum. As always, where did the specimen come from (its locality)? That is important.
16th Mar 2015 11:07 UTCPeter Slootweg 🌟
Do not worry about this specimen. It is a real corundum crystal. The patterns on the crystal faces are very distinctive of this material, the lines intersecting at 90 degrees are due to twinning. The pits are a result of the fact that it grew in a restricted enviroment as most corundum does. The fact that is fluoreses does not mean it is not real. This material is often a mix of red and blue zones. if the reddish zones fluores strongly it will make the complete crystal lit up. The matrix of biotite, feldspar and garnet with this type of corundum is typical for the Zazafotsy quarry in Madagascar where i think your specimen originate.
Peter

16th Mar 2015 13:50 UTCAlfredo Petrov Manager
probably Zazafotsy (or some nearby locality)
The matrix in the second photo is classic, and impossible to synthesize.
But, like Owen, I cannot see how the first photo relates to the second. If you take both UV and daylight photos, it would be much more useful to take them from the same angle.
16th Mar 2015 14:06 UTCOwen Melfyn Lewis
Lamellar twinning in corundum - certainly. But not in two directions at 90 deg to each other, surely? Indication of a 'top surface' cleavage plane? Your comment on possible locality and association with feldspar is very interesting as such 90 deg cross-hatching (cleavage) is not uncommon in feldspar (esp. orthoclase). Like Doug, I have never seen it in corundum
I wonder if we are being misled by the red fluorescence? I cannot relate the fluorescent shape to that of the blue crystal. Are the two one and the same? Further explanation with more images from Joshua might help. (Ed: Crossed with Alfredo's post above).
16th Mar 2015 15:55 UTCReiner Mielke Expert
Maybe that is not a cleavage face but an irregular prism face and what you are seeing at 90 degrees is the same as this: http://www.mindat.org/photo-305989.html
16th Mar 2015 16:00 UTCOwen Melfyn Lewis
16th Mar 2015 16:04 UTCPeter Slootweg 🌟
Regarding the 90 degree crossing lines, these are parallell tot the rhombohedral unit cell. Yes, they are not exactly 90 degrees but it looks like this in the picture. If you orientate a calcite cleavage rhobohedron in the right direction and look at it with one eye it would appear square as well. You are looking at one of the prism faces and to my experience this type of striations on corundum is very common, if not tell tale. Feldspar does have cleavages in multiple directions but not in this orientation.
I'm not sure what you mean by "Top surface cleavage plane". The cleavages run all the way through the crystal and are natural weak spots in the crystal lattice therefore they will be exposed upon the slighttest etching or reabsorbtion. http://www.mindat.org/photo-493506.html is a example from the same locality. The upper crystal also shows these intersecting lines.
I hope it explaines my point of view.
Kind regards, Peter
16th Mar 2015 16:26 UTCOwen Melfyn Lewis

18th Mar 2015 02:45 UTCAnonymous User
Thank you all for your time and inputs sir José, sir Owen, sir Doug, sir Peter, sir Alfredo, sir Reiner, much appreciated.

18th Mar 2015 15:33 UTCOlivier Langelier

19th Mar 2015 01:40 UTCAnonymous User




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 24, 2024 01:03:56
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 24, 2024 01:03:56