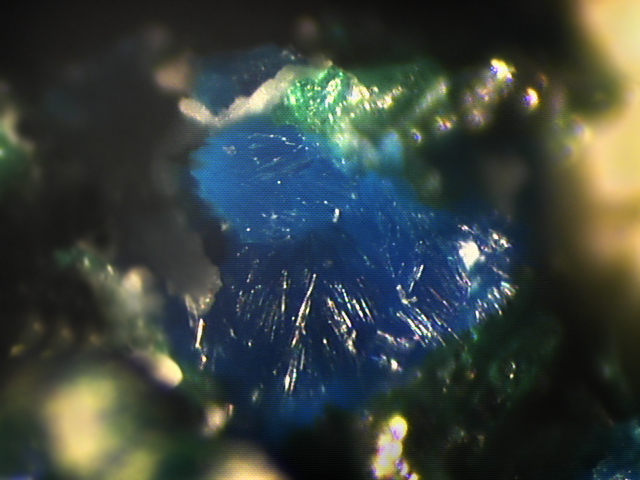
Lemanskiite
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Lemanskiite
Formula:
NaCaCu5(AsO4)4Cl · 3H2O
Originally thought to have 5 waters, and therefore to be a polymorph of lavendulan, but revised to 3 waters by Zubkova et al., 2018.
Colour:
Sky blue
Lustre:
Vitreous
Hardness:
2 - 3
Specific Gravity:
3.78
Crystal System:
Tetragonal
Name:
Named for Chester (Chet) S. Lemanski, Jr. (b. 1947), a New Jersey, USA, collector and former President of the Franklin-Ogdensburg Mineralogical Society, board member and former Vice President of the Franklin Mineral Museum, former hard rock miner (Sterling Mine, NJ) and professional investigator, now retired.
Dimorph of:
Masses and aggregated groups of crystals in and on matrix; massive, filling spaces between breccia fragments. Easy to confuse with lavendulan, with which it may occur simultaneously.
Unique Identifiers
Mindat ID:
9658
Long-form identifier:
mindat:1:1:9658:0
GUID
(UUID V4):
(UUID V4):
ea625561-caa9-4746-bd9c-307a7db2d147
IMA Classification of Lemanskiite
Classification of Lemanskiite
8.DG.05
8 : PHOSPHATES, ARSENATES, VANADATES
D : Phosphates, etc. with additional anions, with H2O
G : With large and medium-sized cations, (OH, etc.):RO4< 0.5:1
8 : PHOSPHATES, ARSENATES, VANADATES
D : Phosphates, etc. with additional anions, with H2O
G : With large and medium-sized cations, (OH, etc.):RO4< 0.5:1
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Lmk | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Lemanskiite
Vitreous
Transparency:
Translucent
Colour:
Sky blue
Streak:
Sky blue
Hardness:
2 - 3 on Mohs scale
Cleavage:
Perfect
(001)
(001)
Parting:
None.
Fracture:
Irregular/Uneven
Density:
3.78 g/cm3 (Measured) 3.86 g/cm3 (Calculated)
Optical Data of Lemanskiite
Type:
Uniaxial (-)
RI values:
nω = 1.749 nε = 1.647
Max Birefringence:
δ = 0.102

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
High
Pleochroism:
Strong
Comments:
O dark green-blue, E light blue-green
Chemistry of Lemanskiite
Mindat Formula:
NaCaCu5(AsO4)4Cl · 3H2O
Originally thought to have 5 waters, and therefore to be a polymorph of lavendulan, but revised to 3 waters by Zubkova et al., 2018.
Originally thought to have 5 waters, and therefore to be a polymorph of lavendulan, but revised to 3 waters by Zubkova et al., 2018.
Crystallography of Lemanskiite
Crystal System:
Tetragonal
Class (H-M):
4 2 2 - Trapezohedral
Space Group:
P41 2 2
Cell Parameters:
a = 9.9758 Å, c = 36.714 Å
Ratio:
a:c = 1 : 3.68
Unit Cell V:
3653.6 ų
Z:
8
Morphology:
Thin tetragonal plates. Crystals bent to the angstrom level.
Twinning:
None mentioned.
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 9.60 Å | (9) |
| 9.18 Å | (100) |
| 4.59 Å | (32) |
| 4.17 Å | (10) |
| 3.06 Å | (15) |
| 2.924 Å | (5) |
| 2.606 Å | (6) |
Comments:
Possibly affected by preferred orientation.
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| 47d : [Arsenates, antimonates, selenates, bismuthinates] | |
| 47g : [Halogen-bearing surface weathering minerals] |
Type Occurrence of Lemanskiite
General Appearance of Type Material:
Rosette-shaped aggregates of thin platey crystals.
Place of Conservation of Type Material:
National Museum of the Czech Republic, Praha, Czech Republic, number P1p 14/99.
Geological Setting of Type Material:
Occurs in the oxidized zone of an enargite-rich gold deposit in an arid environment.
Associated Minerals at Type Locality:
Synonyms of Lemanskiite
Other Language Names for Lemanskiite
German:Lemanskiit
Japanese:レマンスキー石
Simplified Chinese:四方氯砷钠铜石
Spanish:Lemanskiita
Traditional Chinese:四方氯砷鈉銅石
Common Associates
Associated Minerals Based on Photo Data:
| 24 photos of Lemanskiite associated with Lammerite | Cu3(AsO4)2 |
| 4 photos of Lemanskiite associated with Conichalcite | CaCu(AsO4)(OH) |
| 3 photos of Lemanskiite associated with Chenevixite | Cu2Fe3+2(AsO4)2(OH)4 |
| 2 photos of Lemanskiite associated with Lavendulan | NaCaCu5(AsO4)4Cl · 5H2O |
| 2 photos of Lemanskiite associated with Azurite | Cu3(CO3)2(OH)2 |
| 2 photos of Lemanskiite associated with Malachite | Cu2(CO3)(OH)2 |
| 1 photo of Lemanskiite associated with Olivenite | Cu2(AsO4)(OH) |
Related Minerals - Strunz-mindat Grouping
| 8.DG. | Jasonsmithite | Mn2+4ZnAl(PO4)4(OH)(H2O)7 · 3.5H2O |
| 8.DG. | Davidbrownite-(NH4) | (NH4)5(V4+O)2(C2O4)[PO2.75(OH)1.25]4 · 3H2O |
| 8.DG. | Relianceite-(K) | K4Mg(V4+O)2(C2O4)(PO3OH)4(H2O)10 |
| 8.DG. | Pleysteinite | [(H2O)0.5K0.5]2Mn2Al3(PO4)4F2 · 14H2O |
| 8.DG.05 | Lavendulan | NaCaCu5(AsO4)4Cl · 5H2O |
| 8.DG.05 | Sampleite | NaCaCu5(PO4)4Cl · 5H2O |
| 8.DG.05 | Shubnikovite | Ca2Cu8(AsO4)6(OH)Cl · 7H2O |
| 8.DG.05 | Zdenĕkite | NaPbCu5(AsO4)4Cl · 5H2O |
| 8.DG.05 | Rewitzerite | [K(H2O)]Mn2Al3(PO4)4(OH)2 · 14H2O |
| 8.DG.05 | Fluor-rewitzerite | [(H2O)K]Mn2(Al2Ti)(PO4)4(OF)(H2O)10 · 4H2O |
Fluorescence of Lemanskiite
None
Other Information
Thermal Behaviour:
When heated hydrothermally in a quartz capsule at 200°C for 48 hours, it transforms to olivenite.
The DTA curve shows strong endothermic peaks at 70°C and 210°C connected with a loss of a part of the H2O molecules, and small exothermic peaks at 310°C and 520°C.
The DTA curve shows strong endothermic peaks at 70°C and 210°C connected with a loss of a part of the H2O molecules, and small exothermic peaks at 310°C and 520°C.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Lemanskiite
mindat.org URL:
https://www.mindat.org/min-9658.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Lemanskiite
Localities for Lemanskiite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Bolivia | |
| Kempff et al. (La Paz, 2009) +1 other reference |
Chile | |
| Ondruš et al. (2006) |
| Ondruš et al. (2006) |
| Ondruš et al. (2006) | |
Greece | |
| Ondruš et al. (2006) |
| Mintreasure specimen |
| Rieck et al. (2018) | |
Iran | |
| Ondruš et al. (2006) |
Spain | |
| Joan Rosell (2023) |
| Ondruš et al. (2006) +1 other reference |
Quick NavTopAbout LemanskiiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography X-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesCommon AssociatesStrunz-MindatFluorescence Other InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Guanaco Mine, Taltal, Antofagasta Province, Antofagasta, Chile