Efremovite
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Efremovite
Formula:
(NH4)2Mg2(SO4)3
Colour:
Gray, white
Lustre:
Vitreous, Dull
Hardness:
2
Specific Gravity:
2.52 (Calculated)
Crystal System:
Isometric
Member of:
Name:
Named in honour of Dr. Ivan Antonovich Yefremov (Ива́н Анто́нович (Анти́пович) Ефре́мов) (22 April 1908, Vyritsa, St. Petersburg, Russian Empire – 5 October 1972, Leningrad, USSR), paleontologist and geologist at the Paleontological Institute, Moscow. He was also a science fiction writer.
Type Locality:
Mg analogue of ferroefremovite.
A hygroscopic ammonium magnesium sulphate that hydrates to boussingaultite at room temperature.
Compare also Unnamed (NH4-Mg Sulphate Hydrate).
A hygroscopic ammonium magnesium sulphate that hydrates to boussingaultite at room temperature.
Compare also Unnamed (NH4-Mg Sulphate Hydrate).
Unique Identifiers
Mindat ID:
1355
Long-form identifier:
mindat:1:1:1355:6
GUID
(UUID V4):
(UUID V4):
b141cc99-1789-4c3f-93cf-09bd772d4902
IMA Classification of Efremovite
Approved
First published:
1989
Classification of Efremovite
7.AC.10
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
A : Sulfates (selenates, etc.) without additional anions, without H2O
C : With medium-sized and large cations
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
A : Sulfates (selenates, etc.) without additional anions, without H2O
C : With medium-sized and large cations
28.4.4.3
28 : ANHYDROUS ACID AND NORMAL SULFATES
4 : Miscellaneous
28 : ANHYDROUS ACID AND NORMAL SULFATES
4 : Miscellaneous
25.3.16
25 : Sulphates
3 : Sulphates of Mg
25 : Sulphates
3 : Sulphates of Mg
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Efr | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Efremovite
Vitreous, Dull
Transparency:
Opaque
Comment:
Aggregates dull, presumably vitreous as grains
Colour:
Gray, white
Hardness:
2 on Mohs scale
Cleavage:
None Observed
Fracture:
Irregular/Uneven
Density:
2.52 g/cm3 (Calculated)
Optical Data of Efremovite
Type:
Isotropic
RI values:
n = 1.550(1)
Birefringence:
Isotropic minerals have no birefringence
Surface Relief:
Low
Chemistry of Efremovite
Mindat Formula:
(NH4)2Mg2(SO4)3
Crystallography of Efremovite
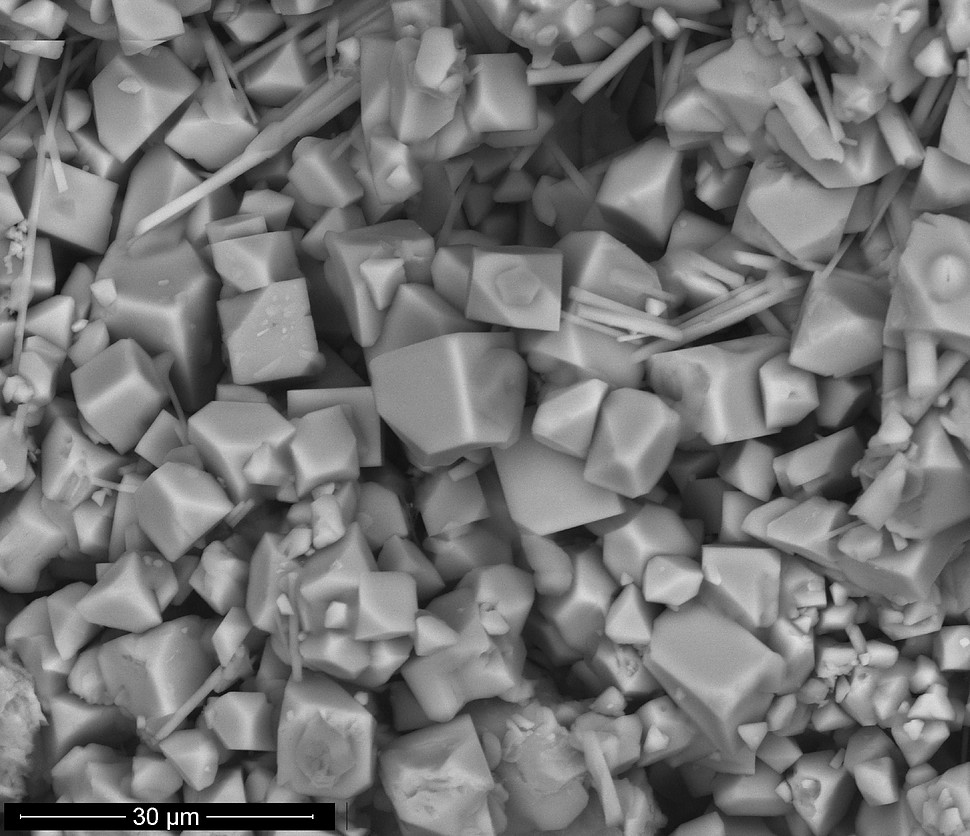
Crystal System:
Isometric
Class (H-M):
2 3 - Tetartoidal
Space Group:
P21 3
Cell Parameters:
a = 9.99(1) Å
Unit Cell V:
997.00 ų (Calculated from Unit Cell)
Z:
4
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 5.76 Å | (35) |
| 4.07 Å | (70) |
| 3.15 Å | (100) |
| 3.00 Å | (35) |
| 2.668 Å | (50) |
| 1.620 Å | (25). |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 45a : [Sulfates, arsenates, selenates, antimonates] | |
| 45b : [Other oxidized fumarolic minerals] | |
| Stage 10a: Neoproterozoic oxygenation/terrestrial biosphere | <0.6 |
| 50 : Coal and/or oil shale minerals | <0.36 |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 54 : Coal and other mine fire minerals (see also #51 and #56) |
Type Occurrence of Efremovite
General Appearance of Type Material:
Equant grains, 0.01-0.015 mm, in gray to white aggregates.
Place of Conservation of Type Material:
Fersman Mineralogical Museum, Academy of Sciences, Moscow.
Geological Setting of Type Material:
From a fumarole at burning dumps at coal mine shafts
Associated Minerals at Type Locality:
Reference:
Shcherbakova, Y.P., Bazhenova, L.F. (1989) Efremovite (NH4)2Mg2(SO4)3—ammonium analogue of langbeinite—a new mineral. Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva: 118(3): 84-87.
Synonyms of Efremovite
Other Language Names for Efremovite
Spanish:Efremovita
Relationship of Efremovite to other Species
Member of:
Other Members of this group:
| Calciolangbeinite | K2Ca2(SO4)3 | Iso. 2 3 : P21 3 |
| Ferroefremovite | (NH4)2Fe2+2(SO4)3 | Iso. 2 3 : P21 3 |
| Langbeinite | K2Mg2(SO4)3 | Iso. 2 3 : P21 3 |
| Manganolangbeinite | K2Mn2(SO4)3 | Iso. 2 3 : P21 3 |
Common Associates
Associated Minerals Based on Photo Data:
| 4 photos of Efremovite associated with Boussingaultite | (NH4)2Mg(SO4)2 · 6H2O |
| 2 photos of Efremovite associated with Pyracmonite | (NH4)3Fe(SO4)3 |
| 1 photo of Efremovite associated with Godovikovite | (NH4)Al(SO4)2 |
| 1 photo of Efremovite associated with Lonecreekite | (NH4)Fe3+(SO4)2 · 12H2O |
| 1 photo of Efremovite associated with Sabieite | (NH4)Fe3+(SO4)2 |
| 1 photo of Efremovite associated with Chabazite-K | (K2,Ca,Na2,Sr,Mg)2[Al2Si4O12]2 · 12H2O |
| 1 photo of Efremovite associated with Quartz | SiO2 |
| 1 photo of Efremovite associated with Tschermigite | (NH4)Al(SO4)2 · 12H2O |
| 1 photo of Efremovite associated with Anatase | TiO2 |
Related Minerals - Strunz-mindat Grouping
| 7.AC. | Aluminopyracmonite | (NH4)3Al(SO4)3 |
| 7.AC. | Amgaite | Tl+32Te6+O6 |
| 7.AC.05 | Vanthoffite | Na6Mg(SO4)4 |
| 7.AC.08 | Pyracmonite | (NH4)3Fe(SO4)3 |
| 7.AC.10 | Langbeinite | K2Mg2(SO4)3 |
| 7.AC.10 | Manganolangbeinite | K2Mn2(SO4)3 |
| 7.AC.10 | Ferroefremovite | (NH4)2Fe2+2(SO4)3 |
| 7.AC.15 | Yavapaiite | KFe(SO4)2 |
| 7.AC.15 | Eldfellite | NaFe3+(SO4)2 |
| 7.AC.20 | Godovikovite | (NH4)Al(SO4)2 |
| 7.AC.20 | Sabieite | (NH4)Fe3+(SO4)2 |
| 7.AC.20 | Steklite | KAl(SO4)2 |
| 7.AC.35 | Aphthitalite | (K,Na)3Na(SO4)2 |
| 7.AC.35 | Möhnite | (NH4)K2Na(SO4)2 |
| 7.AC.35 | Belomarinaite | KNa(SO4) |
| 7.AC.35 | Natroaphthitalite | KNa3(SO4)2 |
| 7.AC.40 | Itelmenite | Na4Mg3Cu3(SO4)8 |
| 7.AC.45 | Saranchinaite | Na2Cu(SO4)2 |
| 7.AC.50 | Majzlanite | K2Na(ZnNa)Ca(SO4)4 |
| 7.AC.60 | Philoxenite | (K,Na,Pb)4(Na,Ca)2(Mg,Cu)3(Fe3+0.5Al0.5)(SO4)8 |
| 7.AC.75 | Petrovite | Na12Cu2(SO4)8 |
Other Information
Thermal Behaviour:
Endothermic effect at 430-495°C, with weight loss of 35.8%.
Notes:
Soluble in water.
In air at ordinary temperatures, the mineral is hydrated to boussingaultite over the course of several days.
In air at ordinary temperatures, the mineral is hydrated to boussingaultite over the course of several days.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Efremovite
mindat.org URL:
https://www.mindat.org/min-1355.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Efremovite
Reference List:
Localities for Efremovite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Czech Republic | |
| Zacek et al. (1995) |
| Hyrsl J. +2 other references |
| Dalibor Matýsek |
| Dalibor Matýsek | |
Germany | |
| in: Stracher +1 other reference |
| T. Witzke & F. Rüger: Lapis 1998 (7/8) |
Hungary | |
| GEODA 2007/1 |
| Szakáll et al. (2008) |
Italy | |
| Russo et al. (2017) |
Japan | |
| Shimobayashi et al. (2011) |
Poland | |
| Parafiniuk et al. (2009) |
| Łukasz Kruszewski (2012) |
| Kruszewski et al. (1 November 2018) +1 other reference |
Russia | |
| Cesnokov et al. (1998) |
| Pekov (1998) |
Tajikistan | |
| D.I.Belakovskiy data |
| Lapis 17 (2) |
Quick NavTopAbout EfremoviteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography X-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Ronneburg U deposit, Thuringia, Germany