Etching Iron Meteorites on the Kitchen Table
Last Updated: 1st Aug 2019By Gareth Evans
Etching Iron Meteorites on the Kitchen Table
The meteorite shown in the accompanying photos was prepared for etching by first deep cleaning using the electrolysis process described in a previous article (see How to Clean Rusty Meteorite - a practical approach). The total cleaning time in this instance was about 3 weeks.
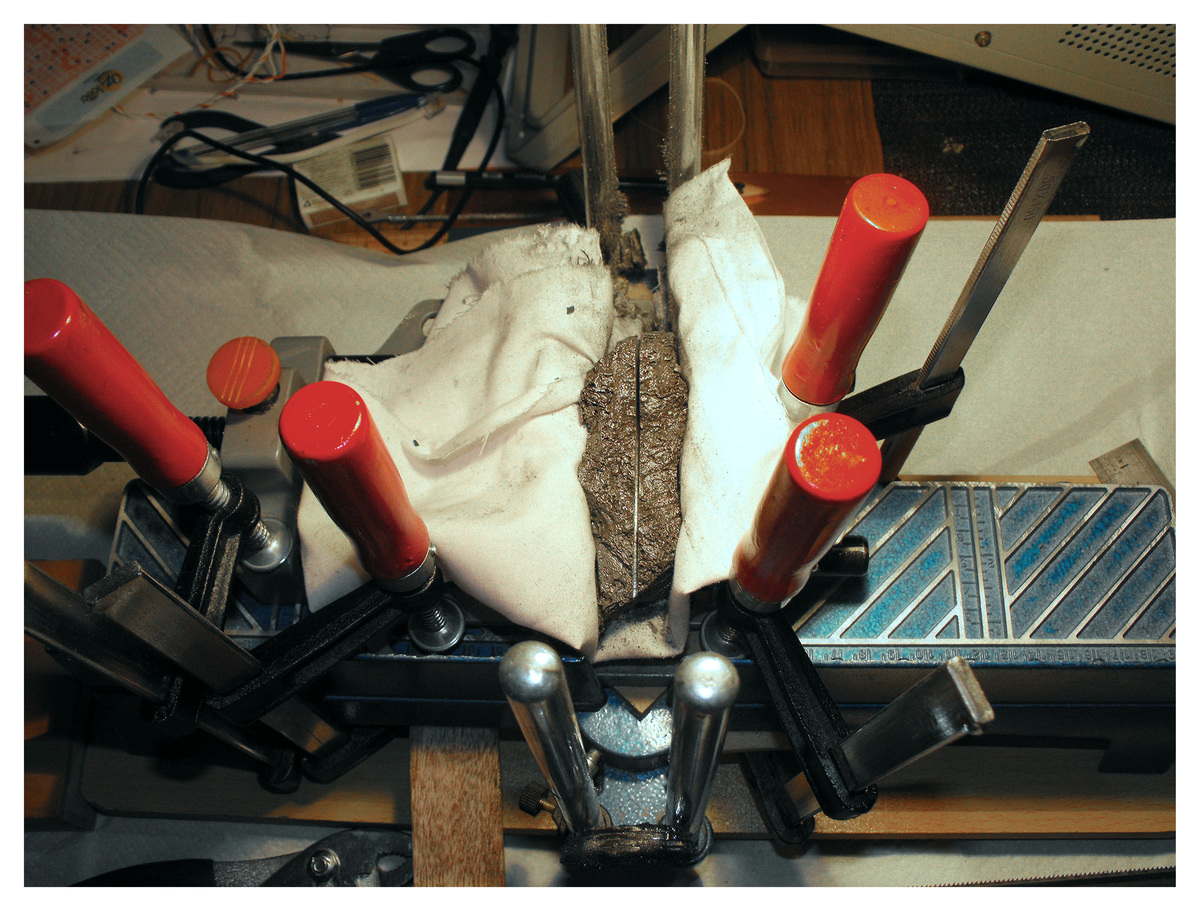
After it had been cleaned it was sliced in half using a bimetallic blade on the kitchen table. It took about 30 minutes of intermittent sawing with the bimetal blade to cut it in half.
The cutting was easy and occurred without incident. This was expected since both Iron and Nickle are much ‘softer’ than the saw blade. In the event of a carbide nodule being present I did have a 1mm diameter diamond wire saw ready. The mitre saw shown in the photo was a cheap $20 import from the local hardware store.
After sawing the slices were polished flat on a granite surface plate using progressively finer grit silicon carbide paper. I started with 120-grit and finished with 3000-grit. When the surface of the slices had a mirror-like luster they were cleaned with dry ethanol and then etched.
Initially I used Nitol, which is a solution of concentrated nitric acid (1 ml) in ethanol (99 ml). I was not happy with the result. The Nitol did not give a deep etch, so I used Ferric Chloride. Ferric Chloride is used to dissolve unprotected copper in circuit board design, and I use it for this purpose when I have no Ammonium persulphate on hand.
The first observation is obvious. You spent three weeks removing chlorides and now you expose the meteorite to chlorides. And this is true, but the exposure time is only two minutes not 500 years, and after etching, the meteorite is left in a 10% solution of sodium hydroxide overnight.
As my dear departed mother (Born Polesworth UK, 1906) would often say, proof of the pudding is in the eating. And the meteorite still shows no sign of rust, and it was etched nearly eight years ago.
The big advantage of Ferric chloride is the end result. It produces sharper Widmanstätten lines with substantially more contrast, and the kamacite plates show stronger Neumann lines than that observed with Nitol. From a practical perspective Ferric Chloride is much easier to purchase than concentrated Nitric acid. You simply brush a light layer of solution on the polished surface and the etching occurs within seconds of exposure to the solution.
What I hope to show is that you do not need a lot of equipment to achieve first class results. With the sweat of your brow and the power of your muscles, you can add value to many ordinary things.
So when you see a ‘nasty’ meteorite, buy it and turn it into a thing of beauty.
The meteorite shown in the accompanying photos was prepared for etching by first deep cleaning using the electrolysis process described in a previous article (see How to Clean Rusty Meteorite - a practical approach). The total cleaning time in this instance was about 3 weeks.
After it had been cleaned it was sliced in half using a bimetallic blade on the kitchen table. It took about 30 minutes of intermittent sawing with the bimetal blade to cut it in half.
The cutting was easy and occurred without incident. This was expected since both Iron and Nickle are much ‘softer’ than the saw blade. In the event of a carbide nodule being present I did have a 1mm diameter diamond wire saw ready. The mitre saw shown in the photo was a cheap $20 import from the local hardware store.
After sawing the slices were polished flat on a granite surface plate using progressively finer grit silicon carbide paper. I started with 120-grit and finished with 3000-grit. When the surface of the slices had a mirror-like luster they were cleaned with dry ethanol and then etched.
Initially I used Nitol, which is a solution of concentrated nitric acid (1 ml) in ethanol (99 ml). I was not happy with the result. The Nitol did not give a deep etch, so I used Ferric Chloride. Ferric Chloride is used to dissolve unprotected copper in circuit board design, and I use it for this purpose when I have no Ammonium persulphate on hand.
The first observation is obvious. You spent three weeks removing chlorides and now you expose the meteorite to chlorides. And this is true, but the exposure time is only two minutes not 500 years, and after etching, the meteorite is left in a 10% solution of sodium hydroxide overnight.
As my dear departed mother (Born Polesworth UK, 1906) would often say, proof of the pudding is in the eating. And the meteorite still shows no sign of rust, and it was etched nearly eight years ago.
The big advantage of Ferric chloride is the end result. It produces sharper Widmanstätten lines with substantially more contrast, and the kamacite plates show stronger Neumann lines than that observed with Nitol. From a practical perspective Ferric Chloride is much easier to purchase than concentrated Nitric acid. You simply brush a light layer of solution on the polished surface and the etching occurs within seconds of exposure to the solution.
What I hope to show is that you do not need a lot of equipment to achieve first class results. With the sweat of your brow and the power of your muscles, you can add value to many ordinary things.
So when you see a ‘nasty’ meteorite, buy it and turn it into a thing of beauty.
Article has been viewed at least 10619 times.