THE s-BLOCK ELEMENTS – A MUSEUM DISPLAY FOR THE HOME OFFICE
Last Updated: 7th Aug 2019By Gareth Evans
THE s-BLOCK ELEMENTS – A MUSEUM DISPLAY FOR THE HOME OFFICE
Introduction
About eleven years ago I embarked on an adventure to create a Periodic Table of the Chemical Elements made from real elements. I wanted to create something special – in a word; unique. This requirement meant I would have to build it myself. First, I had to acquire a few basic hands-on skills – carpentry, metal work, glass blowing and graphic arts.
Why
No doubt you are all aware of my passionate interest in the chemical elements – even the nasty ones! It is hard to put into words the profound significance and importance of the chemical elements. A pious person would have to conclude that the periodic table of the chemical elements is a window into the mind of God. Everything that was (living and inanimate), is and will be is contained in the periodic table of the chemical elements. Reality is truly profound!
The creation of the elements is just as profound. Some formed in the belly of a star long dead. Some elements created from supernova explosions, and other elements created by the collision of neutron stars.
Too often when we collect minerals, and I too am guilty of this, we focus on just how pretty they are, without thinking about what makes them so pretty.
How to
In this how-to I will outline a few steps I took to create one of my very first tables. This table shows the s-block elements (excluding hydrogen) also known as the alkali and alkaline earth metals. I built this table at a time when I had access to some cheap and basic tools. In hindsight I would have preferred to make it out of stained and polished Tasmanian oak – something I might do in the coming months, now that I have some really first-class machine tools.
The photos above show the basic table, made from inexpensive pine bought from the local hardware store. I used a lot of wood screws, too many some might say, but I am a cautious man and I always wear both suspenders and a belt! The table must be structurally sound and very strong.
The photo above shows the decorations being added to the table – architraves – to give it a picture frame appearance.
The above photos show the table sanded with holes filled with wood putty ready for painting, and the painted picture frame. Also included is a photo of the rear of the table which also shows some of the very basic hand tools needed to make it.
Above is a photo of the spacers or shims. These were fabricated from a length of black acrylic from the local plastic supply shop and each shim was cut to size to match the height of the blocks.
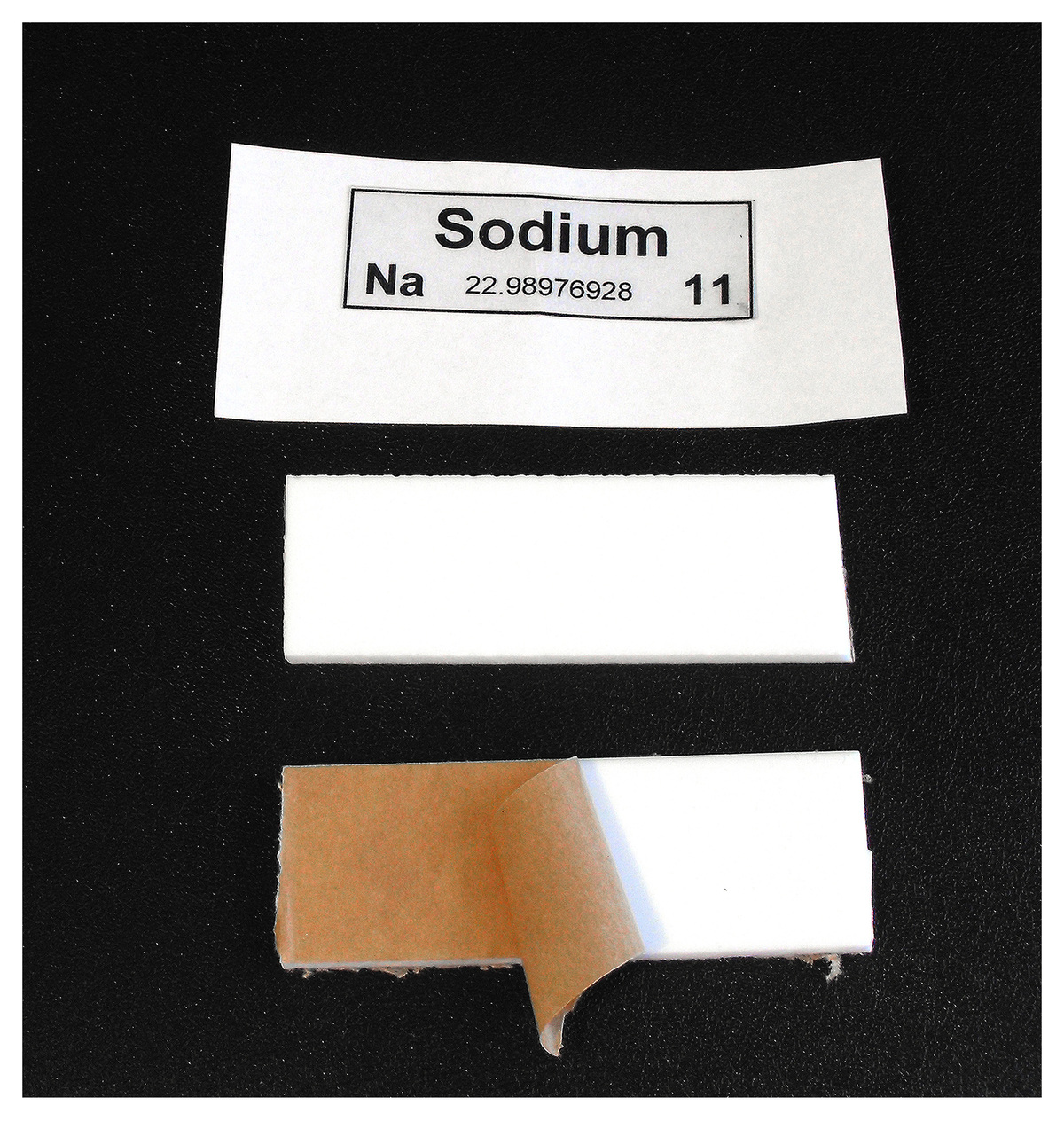
Above is a photo of the material I used to make the labels. They were fabricated from acrylic (white) also from the local plastic shop. To make the labels I used inkjet compatible transparency film which had a sticky side. Once the ink was dry I careful covered the label with a sticky clear plastic sheet – the sort used to protect book covers. Support holes for wood screws were drilled in the white acrylic and the corners rounded.
The above photo shows the completed table (ca. 2013) minus blocks for Potassium and Calcium. Cesium and Rubidium were the only s-block elements I bought that were already sealed in ampoules. I had to fabricate all the other ampoules, and they were made from borosilicate glass test tubes sealed under high vacuum. The blocks are all 100 mm x 10mm x 40 mm in size.
Shown above are the sealed tubes for Lithium, Strontium and Barium respectively. The elements have a distinct metallic luster devoid of any oxidation.
Introduction
About eleven years ago I embarked on an adventure to create a Periodic Table of the Chemical Elements made from real elements. I wanted to create something special – in a word; unique. This requirement meant I would have to build it myself. First, I had to acquire a few basic hands-on skills – carpentry, metal work, glass blowing and graphic arts.
Why
No doubt you are all aware of my passionate interest in the chemical elements – even the nasty ones! It is hard to put into words the profound significance and importance of the chemical elements. A pious person would have to conclude that the periodic table of the chemical elements is a window into the mind of God. Everything that was (living and inanimate), is and will be is contained in the periodic table of the chemical elements. Reality is truly profound!
The creation of the elements is just as profound. Some formed in the belly of a star long dead. Some elements created from supernova explosions, and other elements created by the collision of neutron stars.
Too often when we collect minerals, and I too am guilty of this, we focus on just how pretty they are, without thinking about what makes them so pretty.
How to
In this how-to I will outline a few steps I took to create one of my very first tables. This table shows the s-block elements (excluding hydrogen) also known as the alkali and alkaline earth metals. I built this table at a time when I had access to some cheap and basic tools. In hindsight I would have preferred to make it out of stained and polished Tasmanian oak – something I might do in the coming months, now that I have some really first-class machine tools.
The photos above show the basic table, made from inexpensive pine bought from the local hardware store. I used a lot of wood screws, too many some might say, but I am a cautious man and I always wear both suspenders and a belt! The table must be structurally sound and very strong.
The photo above shows the decorations being added to the table – architraves – to give it a picture frame appearance.
The above photos show the table sanded with holes filled with wood putty ready for painting, and the painted picture frame. Also included is a photo of the rear of the table which also shows some of the very basic hand tools needed to make it.
Above is a photo of the spacers or shims. These were fabricated from a length of black acrylic from the local plastic supply shop and each shim was cut to size to match the height of the blocks.
Above is a photo of the material I used to make the labels. They were fabricated from acrylic (white) also from the local plastic shop. To make the labels I used inkjet compatible transparency film which had a sticky side. Once the ink was dry I careful covered the label with a sticky clear plastic sheet – the sort used to protect book covers. Support holes for wood screws were drilled in the white acrylic and the corners rounded.
The above photo shows the completed table (ca. 2013) minus blocks for Potassium and Calcium. Cesium and Rubidium were the only s-block elements I bought that were already sealed in ampoules. I had to fabricate all the other ampoules, and they were made from borosilicate glass test tubes sealed under high vacuum. The blocks are all 100 mm x 10mm x 40 mm in size.
Shown above are the sealed tubes for Lithium, Strontium and Barium respectively. The elements have a distinct metallic luster devoid of any oxidation.
Article has been viewed at least 2111 times.