Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
PhotosZircon - Jones District, Socorro Co., New Mexico, USA
4th Aug 2017 22:38 UTCMartin Rich Expert
5th Aug 2017 01:53 UTCOwen Lewis
- Not bladed, could be tetragonal.
- Termination good for zircon and not good for brookite.
- FWIW not the usual colour for brookite

5th Aug 2017 02:11 UTCDoug Daniels
5th Aug 2017 02:25 UTCScott Braley

5th Aug 2017 02:33 UTCRolf Luetcke Expert
Wish I could give more information on the piece but I can say, Ron Gibbs, the person I got this from analyzes the material he sells and it came from him.
It seems he found a number of new species for NM over the years he worked there.
Hope this helps a bit.
Rolf
5th Aug 2017 11:51 UTCReiner Mielke Expert
5th Aug 2017 13:51 UTCPavel Kartashov Manager
5th Aug 2017 15:07 UTCMichael C. Michayluk
5th Aug 2017 16:38 UTCUwe Kolitsch Manager
5th Aug 2017 16:41 UTCPavel Kartashov Manager
5th Aug 2017 16:47 UTCDon Saathoff Expert
I don't think I see striations on the XL but rather possible inclusions and I do see (111) faces on the termination - they're small but present.
We've known Ron for nearly 30 years and have not known him to ever make a statement he couldn't back up with good science....
Don
5th Aug 2017 18:03 UTCUwe Kolitsch Manager

5th Aug 2017 18:23 UTCRolf Luetcke Expert
I did look the piece over very well and this is the one good crystal I found on the piece I could even get a photo of. I too had considered it may be somewhere else on the piece but couldn't find anything else what was a good candidate.
I hope Ron will look here and add what he thinks.
Rolf
5th Aug 2017 18:24 UTCDon Saathoff Expert
Another point I failed to make earlier is that when stacking is used a certain amount of fore-shortening of the image takes place - as with a long telephoto lens stopped down for maximum depth-of-field. If the tetragonal XL is angled w/ a "b" face barely showing it will appear to be tabular due to the fore-shortening effect.
I will try to get Ron to comment....
Don

5th Aug 2017 18:40 UTCRolf Luetcke Expert
Here is another angle of the same crystal.
It is not a tabular crystal and has good terminations. It is not striated, as thought earlier but does have inclusions.
There are quite a few bits and pieces of other crystals in the specimen but this is the only complete crystal I could get a photo of.
Rolf

5th Aug 2017 19:29 UTCRolf Luetcke Expert
Here is a different crystal in a pocket, from the side and no terminations are visible on this one, at least not that I can get a photo of.
5th Aug 2017 23:53 UTCDon Saathoff Expert
For those of you who are not native American English speakers we have an expression; "to eat humble pie".....well I'm putting on my bib in preparation. It means that I might have made a mistake and must apologize but I'm not eating just yet.
I haven't heard back from Ron yet but I did receive an email from another ardent micro collector here in Southern New Mexico, Joan Beyer. She said that she had a good number of Jones Camp zircons, She said they were TINY, usually anhedral, occasionally euhedral, and pink (did I mention tiny?). I asked if she could photograph one but she is new to photomicrography and they are tiny!
My bib is in place and I'm waiting with fork in hand.....
Don
6th Aug 2017 00:22 UTCDon Saathoff Expert
Ron sent me a couple of images of the zircons and tomorrow, when I can get to the "real computer" I'll try to upload an image.
Don

6th Aug 2017 00:32 UTCRolf Luetcke Expert
The crystals are actinolite/tremolite and I will certainly fix this and then search the specimen for the zircons that I am sure are on it but I have not found.
Don, from what you said, Joan Beyer has some and they are pink, gives me a starting point.
Thanks all so much for the assist in this.
Reiner, looks like you had it right on at the beginning.
The specimen has at least 5 different species on it and the actinolites were the nicest xls on the piece.
Ron only listed the zircon and nothing else.
6th Aug 2017 00:44 UTCOwen Lewis
-------------------------------------------------------
> Rolf, you may easily to check presence of zircon
> in the specimen under SW UV.
Pavel,
Can you explain? The reference texts I use agree that zircon is most often inert under UVS with occasional weak fluorescence. Reaction under UVL is scarcely any better, the test remaining unreliable. Am I misunderstanding you?

6th Aug 2017 01:29 UTCRolf Luetcke Expert
After a lot of study of the piece, this is the only actual zircon I found that is free standing. Others are broken.
This is why mindat is such an important site. I had looked at the location and no photos of the actinolite on there and zircon was not even listed.
People do seem to know it is there and what it looks like but with no photos one is often in the dark with figuring out what the mineral from that location looks like.
I do so much appreciate the help from all on mindat with this.
I did try the UV response but since the crystal here is about 1/2mm, it didn't do anything.
I will add the "real" zircon to the site and now hopefully both species will at least have one photos.
Hope a few of the others who have specimens can also add photos.
Thanks all
Rolf
6th Aug 2017 01:29 UTCMartin Rich Expert
6th Aug 2017 01:39 UTCPavel Kartashov Manager
I don't know which reference texts you used, but my own personal expirience shows, that in many magmatic rocks zircon is the most bright fluorescenting mineral. And from 100 zircon specimens only 2-3 will be nonfluorescent (usually this is indication of deep radiational damage of mineral - typical for cyrtolites). The most usual color of fluorescention is yellow-orange (caused by oxygen defects on incorporated Fe3+ ions), but I saw green, white, pure yellow, light-blue and pinkish fluorescention of zircons. But, of course, very specific spoty yellow-orange glow is absolute leader - 90 cases from 100.

6th Aug 2017 01:44 UTCRolf Luetcke Expert
Thanks for your input also on this. I did try on the fluorescence but the actual zircon is so tiny that the UV light didn't have any response. I do have a good light but no response on this specimen.
Thanks for all the help.
Rolf
6th Aug 2017 02:29 UTCPavel Kartashov Manager
I don't see any sence to have in collection zircons of such size as this from irgizites of Zhamanshin crater.
Should I to include it into locality mineral list? ;-)
Zircon of smallest size appreciated in my collection is such rich impregnation of blue tin-bearing zircon micro-xls within quartz from Colorado. Q?uartz masses are coloured in blue by this natural heated starlite.
But usual zircon sample here looks like this 1.6 cm long doubleterminated monoclinic in shape xl from Ilmeny Mts.

6th Aug 2017 03:48 UTCDoug Daniels

6th Aug 2017 04:48 UTCAlfredo Petrov Manager
6th Aug 2017 17:33 UTCPavel Kartashov Manager
6th Aug 2017 20:23 UTCOwen Lewis
Zircon is truly fascinating stuff. Even presence/absence of the UV component in sunlight can be sufficient to radically alter the perceived colour of a crystal - as can heating as mild as 150 deg C in some cases. In this case, I don't buy an 'its a gem/mineral thing'; the science is pure here and the same for both groups in this case.
A pre-eminent mineralogist (PhD Mineralogy Harvard) and gemmologist, Joel Arem, wrote the following on luminescence in Zircon which, as a general, short statement, I don't think can be bettered, "The fluorescence of zircon is variable. Some material is inert. Other crystals glow intensely".
Of the eight gem-grade zircons in my personal collectionsix are cut and range from high (4) to metamict (2) in character. Sizes vary from one to twenty ct. Two (not metamict) are uncut showing different crystal habits. Illuminating at 365 nm and 254 nm gives the following results:
- Two metamicts. Inert and inert
- Two uncut. One weak LW and moderate SW. One weak and weak.
- Four cut. Two inert and inert. One weak LW and moderate SW. One weak LW and very weak (am I dreaming this?) SW.
With reference to Pavel's last post above:
- Metamicts that are heat treated are no longer metamict.
- Of my two uncut crystals, one only is weakly fluorescent and both have the typical reddish-brown colour of unheated Sri Lankan material.
- Of the four cut high zircons, 1 x colourless 20ct, 1 x golden, 1 x red & 1 x hyacinth, two show some fluorescence and two show none. None show signs of heat treatment (severe for colourless).
- The standard work on gemstone enhancement was written Kurt Nassau, another PhD, chemist, mineralogist and gemmologist. He does not mention any causal link between heat treatment in zircon and absence of fluorescence. He ddid however have a passing thought that there might be linkage between the metamict process and lack of fluorescence. See more in last paragraph.
I think one can fairly conclude from all the above that UV fluorescence is an unreliable test for zircon. Perhaps that is why my preferred source of optical information on gem materials, Birgitte Guether's 'Bestimmungstabellen' does not include *any* UV testing results for zircon :-) Another seven works of reference on my shelf indicate that testing is likely to give mixed results. For all to see, there is also http://www.gemsdat.be/zircon.htm This lists the results obtained in over 40 independent tests either carried out by GIA staff or published by them over course of 20 years or so.
If I have learned anything, it is that no single tabulation of results to be expected in material testing should be taken as gospel. Rather, rely on information that ((say) six such experienced researchers report. Note but treat with caution outlier results reported only by one source. I could name (but won't) a couple of authors who are 'notorious' for reporting, time and again, outlier test results that no one else seems to find.
But returning to zircon and fluorescence. I do wonder if the erratic fluorescence test results that are clearly to be found might be related to the degree of damage to the crystal structure through the metamictisation process. But though such speculation is all very well, to make a statistically strong case would require some careful crystal grading and perhaps the testing of some 200 pieces of so in total?
Edited to incorporate response to Pavel's last post. And tidy.

6th Aug 2017 23:11 UTCDoug Daniels
Owen-
Sounds like a Master's project for some young lad/lass. Likely won't ever happen, but....
7th Aug 2017 13:07 UTCOwen Lewis
7th Aug 2017 14:19 UTCPavel Kartashov Manager
we speak on different languages apparently about different things.
I live with zircons from 1975. I see them practically every day of my life. Zircons were presented in every object, which I investigate (some decades my specialization was REE-Nb&Ta-Zr deposits) or collecting. One time in my childhood I collected zircons by their localities, but stoped to do this specially on number 86. I know zircons as they are in nature (on the Earth and on the Moon). I saw billions of zircon crystals from micros of nanometer-size up to fist-size chunks.
I wouldn't to say that I know all about natural zircons, but they composed (and still composing) big part of my life.
I am able to tell you a lot of stories about zircon. Look like, this for example. Whole such rocks https://www.mindat.org/photo-142966.html from this locality (practically whole this https://www.mindat.org/photo-155688.html mount) fluorescent in UV by typical orange-yellow colour of zircon. These contact hornfels (or low-alkaline fenites) are penetrated by microcrystalline zircon. Any 0.3 mm grain of quartz or feldspars forming these rocks contains inside 5-20 inclusions of 10 mkm-size zircon crystals. They works as 5-20 light-emitting diodes placed into children air balloon filled with fume. Whole volume of transparent albite lighting by weakened colour of zircon fluorescence. Lazer fluorescence of such large objects is visible from near-Earth orbit, from space.
You have expirience of handling with a few cut stones (half of which presumably were treated) and reading of special gemmological literature.
Our statements are based on slightly different backgrounds, don't them? ;-)
7th Aug 2017 16:51 UTCUwe Kolitsch Manager
7th Aug 2017 19:09 UTCOwen Lewis
Pavel Kartashov Wrote:
-------------------------------------------------------
> Hi Owen,
> we speak on different languages apparently about
> different things.
True. Had I to write in Russian... well, this conversation would not be taking place :-) But I do know a little Russian history - and particularly enjoy Sergei Eisenstein's films 1925 - 1928 and 1937 - 1945.
As I understand it, you implied that UV fluorescence is always present under test if the material is primarily zircon. If I have misunderstood your meaning, please do correct me. The fact remains that, whatever your personal observations may have been (or mine may have been), there is general agreement that UV fluorescence in zircon is entirely unreliable. See Arem quoted earlier. This is not a matter of opinion but of public record.
Whether rocks that contain some percentage of zircon may fluoresce is a different matter and (I'll guess ;-) ) has no single answer for all possible combinations to be found. It is in any event an irrelevance as the thread concerns itself not with a rock composition but the possible ID of a millimeter-scale crystal.
I think we may have to agree to disagree.
7th Aug 2017 22:40 UTCPavel Kartashov Manager
I do not intend to bicker with you. Belive to your "public redcords" and lay up your personal experience.
I did not engage to teach you.
Of course, as told my teacher O.V. Kononov https://www.mindat.org/photo-792559.html - a color of mineral is the most treacherous its diagnostic property.
I would add to this - UV fluorescence is even more treacherous property than color. I saw nonfluorescent willemite ( https://www.mindat.org/photo-691612.html ) and scheelite (https://www.mindat.org/photo-72577.html ). So, of course, you may to meet nonfluorescent zircon too. And this will be much more easy, than to meet nonfluorescent scheelite.
But if you'll look at this nonfluorescent zircon it turned out or metamict (cyrtolite), or hydrated (malacon), or heated/annealed (all starlites and some colorles or light-colored samples).
When cut zircon is nonfluorescent, this is for me direct indication, that the specimen underwent a thermal treatment.
Usual accessory zircon from granites, gneisses, syenites and alluvial/marine placers has bright and very typical yellow or orange-yellow UV fluorecence. In constancy of this property zircon is comparable with willemite.
What about size. Micro-grain of zircon of 10 mkm size give quite enough light output to be well visible under binocular. ;-)
This is the latest replenishment of my zircon suite, arrived to me right today - 2 cm size doubleterminated polychrome prism.
:-)
8th Aug 2017 05:45 UTCDean Allum Expert
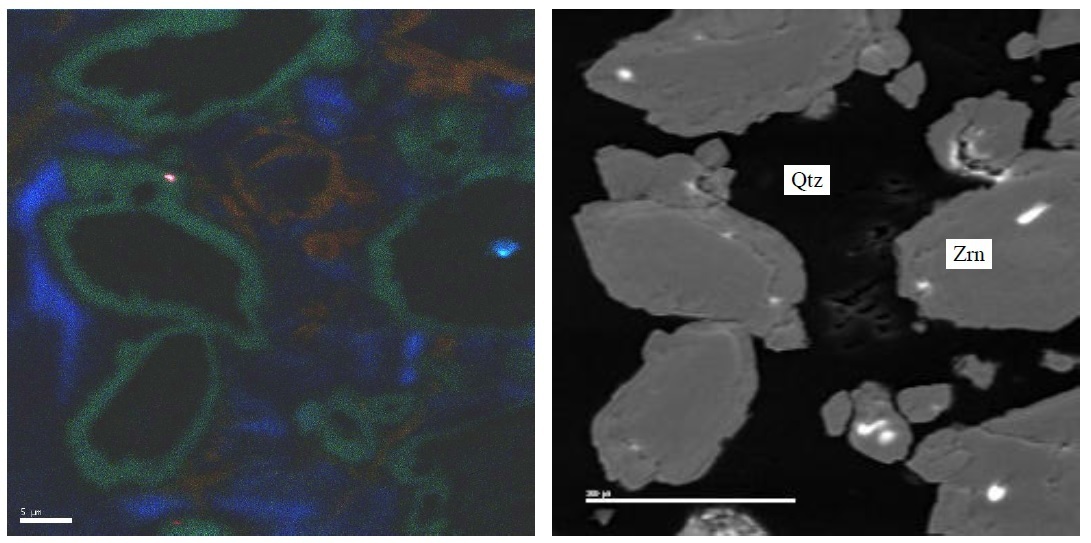
FYI, Even that blue hydrothermal zircon shows some "flourescence" with the cathodoluminescence method. (CL on left, SEM on right)
Because most zircons are igneous and form with the same other elements, AND are conveniently exceedingly INERT, they should all share similar optical properties. You have already mentioned the radiation exception. If they do not fluoresce, you are not looking hard enough. It seems that an industry has been built around zircon dating because of it's uniformity.
Regards,
Dean
8th Aug 2017 14:13 UTCPavel Kartashov Manager
glad to hear from you!
Of course UV isn't the single source of excitation of fluorescention - it is only most accessible and simple. Many minerals being inert under UV are glow under another source. For example cassiterites and kyanites able to have bright catodoluminescention.
One girl in our Institute made his PhD on fluorescention of sphalerites, rutiles etc.
Besides UV we have X-ray, electron beams, lasers. I was very surprised to see fluorescention of chlorargirite under laser beam. These guys made mobile car-based installation for field searchings of silver aureoles of scattering around polymetallic deposits for Far East. They also works on similar cosmic-based equipment.
9th Aug 2017 02:55 UTCOwen Lewis
-------------------------------------------------------
> Pavel Kartashov Wrote:
> --------------------------------------------------
> -----
> > Rolf, you may easily to check presence of
> zircon
> > in the specimen under SW UV.
>
> Pavel,
> Can you explain? The reference texts I use agree
> that zircon is most often inert under UVS with
> occasional weak fluorescence. Reaction under UVL
> is scarcely any better, the test remaining
> unreliable. Am I misunderstanding you?
Pavel Kartashov Wrote:
-------------------------------------------------------
> Owen,
> I do not intend to bicker with you. Belive to your
> "public redcords" and lay up your personal
> experience.
> I did not engage to teach you.
Nor I to read an exercise in incivility.
Pavel Kartashov Wrote:
-------------------------------------------------
> .....Of course, as told my teacher O.V. Kononov
> https://www.mindat.org/photo-792559.html - a
> color of mineral is the most treacherous its
> diagnostic property.
And another (wiser?) man added, 'But only a fool ignores it' ;-) Colour always tells us something. Whether or not that 'something' is useful for some purpose in hand we can agree is another matter. Frequently it is inconsequential - but it is not always so. Colour is only a construct of the human mind - an analogue used by mankind to describe, store and communicate information about energy radiated in one very narrow band of the e-m energy spectrum.
> I would add to this - UV fluorescence is even more
> treacherous property than color.
> ..... So, of course, you may to meet nonfluorescent
> zircon too. When cut zircon is nonfluorescent, this is
> for me direct indication, that the specimen underwent
> a thermal treatment. Usual accessory zircon from
> granites, gneisses, syenites and alluvial/marine placers
> has bright and very typical yellow or orange-yellow UV
> fluorecence.
Yet all zircon occurring in (e.g.) igneous rock has been heated most severely at least once in its life?
We are surely missing something here. All agree that metamicts are inert under UV - but not all zircon is either metamict or is somewhere along the road to becoming metamict.
Does anyone know of a study relating UV fluorescence in zircon to chemical composition of a significant number of samples? For example, it has been known for many decades that Al2O3 is inert under UV. However, with trace amounts of Cr in substitution for some of the Al (around 1%), the material becomes red rather than colourless and is strongly red fluorescent under UV. Unless Fe is also present in trace amount in the crystal lattice. Where Fe is so present, even at a few hundredths of a percent, then the crystal remains red in colour but the strong red fluorescence is either greatly diminished or extinguished entirely (according to Fe level). Does anyone have access to a paper on this matter concerning zircon that they can link to here? FWIW, heating ruby up to and beyond the piont of melting does diminish the UV fluorescence of the cooled material....
It seems that we are, finally, agreed also that inert/weak UV reaction in zircon does occur. It follows therefore, (just as for many other minerals) the testing of zircon for fluorescence under UV with unaided visual inspection is - at its best - indicative and never can be diagnostic. The reason for this to my mind likely lies, as for ruby, in the inhibiting effect on the UV fluorescent reaction when (as is almost always so in crystals produced under geological conditions) there are impurities present. But that remains to be proved.
@Dean,
Nice and interesting post but not a lot to do with UV fluorescence in zircon;-). A paper I have on the technique to which you refer (Shi, G., et al., Age constraint on Burmese amber based on U-Pb dating of zircons, Cretaceous Research (2012), doi:10.1016/j.cretres.2012.03.014) implies the use of cathodoluminescence to assist in the isolation of zircon grains rest of the matrix in which the amber was entombed. A decent number of such grains were removed and prepared for radio dating after careful calibration against a Plesovice standard zircon. For the radio dating, traces of U, Th and Pb were used, advancing significantly the accuracy of earlier work carried out on the dating of burmite.

9th Aug 2017 03:44 UTCDoug Daniels
9th Aug 2017 04:08 UTCDon Saathoff Expert
Don
10th Aug 2017 01:49 UTCDon Saathoff Expert
Don

10th Aug 2017 03:48 UTCDoug Daniels
10th Aug 2017 10:43 UTCOwen Lewis
10th Aug 2017 12:54 UTCPavel Kartashov Manager
Crystals of free growth in cavities (pneumatolitic or hydrothermal origin) in nonalkaline rocks isn't the most usual position for zircons. So different variants still are possible.
10th Aug 2017 14:19 UTCOwen Lewis
10th Aug 2017 15:17 UTCPavel Kartashov Manager
10th Aug 2017 17:42 UTCPavel Kartashov Manager
10th Aug 2017 19:06 UTCDon Saathoff Expert
The various phases of the dike occassionally have miarolitic cavities containing well-formed microcrystals of minerals of interest to mineral collectors. These include titanite, actinolite, apatite and zircon......."
I'm still waiting on someone who has a powerful enough SWUV to reach into the cavities under the 'scope and add data about fluorescence......patience, Owen & Pavel!!
Don

11th Aug 2017 01:18 UTCDoug Daniels
12th Aug 2017 22:08 UTCOwen Lewis
-------------------------------------------------------
....
> I'm still waiting on someone who has a powerful
> enough SWUV to reach into the cavities under the
> 'scope and add data about
> fluorescence......patience, Owen & Pavel!!
>
> Don
I look forward to seeing the results :-)
It seems that chemists have done a fair amount of academic study of the quenching of fluorescence. There are various ions that have this quenching property and, of these, Fe3+ is particularly common and its quenching effect well documented. There is no disagreement that zircon is frequently found to fluoresce under UV. My only concern is with thought such as:
- Fluorescence under UV is 'diagnostic' for zircon. 'A supportive observation' certainly. IMHO, this is true for other fluorescent minerals as well. Because UV fluorescence is so often quenched, no certainty of specimen ID can be drawn from either its presence or absence.
- That the absence of UV fluorescence in zircon assured that there has been anthropogenic heat treatment.
The older I get, the less place I seem to find for absolutes... Never mind; its good to think about things afresh from time to time.
12th Aug 2017 22:40 UTCDon Saathoff Expert
And, like you, Owen, I've reached an age when I realize the absolutes of my youth tend to evaporate like so much smoke....
Don
13th Aug 2017 01:51 UTCPavel Kartashov Manager
-------------------------------------------------------
> There is no disagreement that zircon is frequently
> found to fluoresce under UV. My only concern is
> with thought such as:
> - Fluorescence under UV is 'diagnostic' for
> zircon. 'A supportive observation' certainly.
> IMHO, this is true for other fluorescent minerals
> as well. Because UV fluorescence is so often
> quenched, no certainty of specimen ID can be drawn
> from either its presence or absence.
Is green fluorescention of willemite diagnostic? Or it is "diagnostic"? :-))) We have a lot of fluorescenting willemite, but we have and nonfluorescent examples.
Zircon is very wide spreaded mineral. It is among the 50 minerals that any geologist must recognize.
Diagnostic of zircon (similar to any other minerals) based on complex of mineral properties: its outer appearance - color, lustre, transparency/nontransparency, morphology of crystals, its physical properties - hardness, specific gravity, magnetism, radoactivity, capturing of neutrons, fluorescence, its chemical properties - solubility in different reagents, specific element reactions, its geological setting - type of rock and mineral association, its interaction with different rays - X-rays, electrons, laser beams.
So fluorescence isn't the single (and, of course, not the main) diagnostic property of zircon (or any other mineral). I don't know at all any mineral fluorescention of which able to be its single and main diagnostic property.
But some minor properties of minerals able to help us fast to find their small inclusions in rocks. Particularly fluorescention of zircon in UV. Or its radioactivity if you use alpha-radiography. Or typical levels of neutron capturing if you have necessary equipment.
Who talks about absolut here? Definitely not me.
Owen Lewis Wrote:
-------------------------------------------------------
> - That the absence of UV fluorescence in zircon
> assured that there has been anthropogenic heat
> treatment.
Most of natural zircons has too deep coloration to be used in jewellery directly. Being transparent and nice colored in small (0,n-1 mm) grains, they become translucent and deep coloured in grains of larger (fit for cuting) size.
Heating able significantly reduce coloration of zircon and to make it usable for jewellery. So heating of zircons became usual procedure of treatment of them from ancient times.
May be 95-99% of natural zircon crystals (especially of large sizes) needs in heating before faceting. And they are treated.
As we knows, hetaing destroys florescent centers within zircon structure. So absence or reduction of UV fluorescence is diagnostic property very sensitive to such treatment.
If you have facetted zircon with strong UV fluorescention, this means that you have completely natural unheated zircon. It is exceedingly small probability, of course. You should to have 1000 or more facetted zircons from different producers to be happy to meet such rarity.
Because of zircons are treated in mass quantities, producers aren't interested in wide distribution of information about almost total heating of zircons for jewellery.
The another consequence of this is comparatively higher abundance of UV active specimens within small facetted zircons in comparison with larger ones.
You may to take cheap, colored nontransparent zircon with strong UV fluorescention and anneal it on gas stove. After heating you'll obtain more light-colored and weak fluorescent crystal.
I do this myself in my childhood, transforming brown zircon crystals into honey-yellow, more transparent (but much more craked) ones.
If you will slowly heat in special electric furnace and then even more slow cool in it such crystal http://www.ebay.com/itm/RARE-Gem-Quality-ZIRCON-large-crystal-from-Seiland-Hammerfest-Norway-/322608803241?hash=item4b1cfbada9:g:5qoAAOSwfjhZdFYy you probably will obtain material with some zones fit for facetting of couple perfect large hiacinth stones.

13th Aug 2017 03:25 UTCDoug Daniels
13th Aug 2017 09:24 UTCJoel Dyer
I've also been wondering what the exact connection to zircons' different types of photoluminescence is and whether someone will carry out some very extensive studies on this subject.
I've ran tests on both metamict, semi-metamict and seemingly quite fully intact zircons, and I don't quite understand how zircons' photoluminescence works.
There doesn't seem to be an exact connection at least to 532nm laser "Raman" photoluminescence peaks vs. UV-fluorescence. I would also venture - as a non-expert - that perhaps high metamictness lowers the UV fluorescence, depending on case?
Could it be, that when the REE content- some of which has been radioactive - becomes to high, the UV luminescence is weakened, but the Raman & other photoluminescence is much stronger?? I simply can't quite understand this...
Here's an example of a fractured, but nicely formed zircon that had almost nonexistent photoluminescence under laser and quite noticeable SW and mid-weak LW UV fluorescence. The width is about 7mm Many zircons have considerable photoluminescence peaks when using 532nm laser. In case someone is wondering, the baseline correction for this sample was very minimal, thus no PL peaks have been "wiped out" by any optimization.
Sorry for the poor-quality UV picture, I shot this handheld this time, with extension rings on the camera and of course the hands shook....The UV fluorescence should be a little bit stronger than shows here.
And here's the data after heating the zircon up to 1000 degrees celcius. The heating seemed to strengthen both some Raman peaks and visually the UV fluorescence and add some PL peaks.
Joel
14th Aug 2017 17:34 UTCOwen Lewis
Yes, understood and in general I agree. The problem is that the development of an informative exchange of posts and/or the development of a train of thought is not always a tidy business and tends to occur in forum postings as, when and where the spark lights. If the matter is thought sufficiently important, I suppose that a mod could always move or duplicate such content into a more appropriate area - but its all work and in most cases probably not worth the effort?
@ Joel,
Wow! The tale told in pics. I envy you your raman set-up. If one of the speculative investments I make to top up my pension income comes good next year (and with my health no longer being good enough to really enjoy a round the world cruise), out of my winnings I shall buy myself a Raman/FTIR rig and open the door to several years of new investigations and study. But I digress (again!).
Your photos and graphs are excellent and thought-provoking. It's great to have such positive confirmation from another that the anthropogenic heating of zircon crystals does not necessarily destroy (and may even enhance) the fluorescent quality. QED. A cut and paste copy of your post is added this day to my little archive of information on gem properties. Would you be kind enough to mail me copies of those images, so I can look at and store them at their highest quality?
What you show is of course what one should predict for any zircon that, as it comes from the ground has Fe3+ trapped in the crystal lattice. When strongly heated, an Fe3+ accessory changes to an Fe2+ accessory and, in doing so, loses much/all of its ability to quench fluorescence.. Thus a zircon crystal such as yours can fluoresce more strongly after heating than it could do before. This is, of course, not only true for zircon but also ruby, sapphire, emerald, heliodor, aquamarine, green beryl, alexandrite and more. I use gem varietal names here rather than the mineral species names because the fluorophore in corundum, beryl, chrysoberyl (and others is an accessory ion Cr2+ rather then the essential mineral.
This seems to explain, at least in significant part, the uncertainties of outcome in a simple test for UV fluorescence in a random selection of zircon crystals heated and unheated (as teported earlier in this thread for my own little reference collection eight assorted zircon crystals).
Thanks again.
14th Aug 2017 17:53 UTCPavel Kartashov Manager
From other hand, Fe3+ center itself is source of fluorescention in zircons on some small level of concentration. Particularly in colorless zircons from kimberlites of Yakutia.
15th Aug 2017 22:20 UTCOwen Lewis
In direct answer to your question, the traditional method of 'burning' zircon to manipulate its colour is in a very basic charcoal brazier, which gives the reducing atmosphere (CO) required. The duration of heating etc. is determined by trial and error, varying between different lots of untreated zircon. But you must know this, surely?
In the matter of fluorescence and its quenching, there is no simple single solution that covers all cases. Dr Kurt Nassau defined all the causes of causes of colour as a short list of fifteen. Of these fifteen, any one of four of them may cause fluorescence, depending on the material and environmental factors. These four are:
- For transition metal compounds, ligand field effects.
- For transition metals present in trace quantities in a crystal, ligand field effects also.
- In some organic compounds, transition between molecular orbitals.. Charge transfer causes colour in some minerals but does not, according to Nassau, cause fluorescence in minerals.
- Transitions involving energy bands, such as cause the formation of colour centres, may also cause fluorescence.
There is no single mechanism whereby the quenching of fluorescence occurs. Rather, from memory, there are four or so different mechanisms. And, to top off this heap of variables and complexities, some fluorophores will change to act as a quencher if the at wt% present falls outside certain limits. Those limits vary according to the material. A particularly well-known example of this is the Cr2+ cation. Fe3+ is another I think. There are at least eight metal cations (including Fe3+) that can, in the right circumstances, act to quench fluorescence.
Finally, all that we have covered so far does not (AFAICS) explain why metamict zircon crystals are never found to fluoresce :-/
Turning back then to Joel's zircon heating test and his results. At this point, none of us can know what fluorophore or combination of fluorophores there are in the crystal he heated and so decisively showed that post-heating the fluorescence had actually increased, contrary to the opinion of your good self and, no doubt, of others as well. Similarly, we do not know whether Fe3+ is present in the correct quantity to act as a quencher. Nor do we know the at wt% of its presence, if indeed it is present at all. All that can be said for certain is:
- Fe3+ is the most common of the metal cations that commonly can act as fluorescence quenchers.
- Fe3+ is commonly an accessory in zircon (and other) crystals.
- The weak UV fluorescence shown by Joel's unheated specimen and stronger fluorescence after heating is exactly what you should expect if Fe3+ was present pre-heating.
Enough said?

15th Aug 2017 23:00 UTCAlfredo Petrov Manager
I would argue that "metamict zircon" is an oxymoron and is not zircon at all because, by definition, it does not have a zircon crystal structure. For convenience, metamict minerals are often considered to be "varieties" of their parent species, but in reality they are completely different and so there is no particular reason why they should be expected to show the same fluorescence characteristics.
16th Aug 2017 01:09 UTCPavel Kartashov Manager
-------------------------------------------------------
> Pavel,
> In direct answer to your question, the traditional
> method of 'burning' zircon to manipulate its
> colour is in a very basic charcoal brazier, which
> gives the reducing atmosphere (CO) required. The
> duration of heating etc. is determined by trial
> and error, varying between different lots of
> untreated zircon. But you must know this, surely?
Yes I know this of course. :-) I know even more. Particularly in old times on Ural, raw zircon crystals baked in dough to obtain hyacinths.
But why (from which infoirmation) you decided, that Joel used "very basic charcoal brazier" for heating of exactly his specimen up to 1000oC? It is enough difficult to obtain such temperature in simple charcoal brazier. I am suppose, Joel used electric muffle furnace. In other words annealing took place in usual atmospheric air. ;-)
You are a wonderful theoretician. But your theorizing does not explain simple and obvious facts. In particular, the bright luminescence of the vast majority of crystals of natural zircon.
Rid me of quoting textbooks on quantum physics and optics. I just drew your attention to the fact that your personal observations are at odds with what we observe in nature on a much larger number of samples. Only that and all.
16th Aug 2017 04:45 UTCOwen Lewis
1. I make no claims/assumptions for what Joel did or did not do in the heating of his zircon or for the accessory(ies) in his specimen, though you state that I have.
2. Fe3+accessory is a likely fluorescence quencher in many cases, simply because its occurrence is almost ubiquitous.almost any situation, where the at wt% is within the correct limits.
3. I do observe that the result Joel obtained is in accordance with expectation when a quenching accessory is reduced/eliminated as a consequence of strongly heating the specimen. Your earlier posts advised that this is an effect that you do not believe happens in zircor. If you now have a better explanation for Joel's observations, I'm sure that some of us will read that with care and interest.
4. Others whose curiosity has been piqued by this protracted exchange may observe the UV fluorescence or lack of it for themselves in their own collections of zircon and draw their own conclusions as to the reliability (or lack of it) of a show of UV fluorescence in zircon. In any event, it has been shown here in two small but independent and quite random tests that your assertion that non-fluorescence in zircon is a reliable indication that the material has been heated does not stand up.
5. What do you mean by 'a vast majority of zircon samples'? >99.99%? > 55%? of a statistically significant and randomised sampling? If you have the results from such testing, it would be useful indeed, for the guidance f others, to see it written into the record here along with a good description of the methodology used, sources of origin etc. If a proper sampling is not too 'theoretical', that is.....
But thanks for encouraging an an old man to 'stretch his legs'. It still feels good ;-)

16th Aug 2017 04:55 UTCLawrie Berthelsen (2)
The gem quality pieces that we dug ranged from brown, violet, pink, yellow, and colourless, but none of them fluoresced. I have often wondered "Why is it so?"
Lawrie, F.G.A.
16th Aug 2017 05:12 UTCOwen Lewis
-------------------------------------------------------
> "...explain why metamict zircon crystals are never
> found to fluoresce"
>
> I would argue that "metamict zircon" is an
> oxymoron and is not zircon at all because, by
> definition, it does not have a zircon crystal
> structure. For convenience, metamict minerals are
> often considered to be "varieties" of their parent
> species, but in reality they are completely
> different and so there is no particular reason why
> they should be expected to show the same
> fluorescence characteristics.
You are right Alfredo, but what is one to do? Sidestep the linguistic trap by writing 'metamict ZrSiO4'? [Ed: Tsk...] I think fluorescence or the lack of it play out according to the rules of chemical structure rather than mineral nomenclature?
Life's too short ;-) Please forgive.
16th Aug 2017 05:39 UTCOwen Lewis
-------------------------------------------------------
> For what it's worth - some years ago I was
> fossicking at the famous Australian zircon
> locality of Mud Tanks in the Northern Territory.
> My friend had a portable SW UV lamp, and it was
> with much excitement that we first went out at
> night expecting to find our fortunes under UV.
> Well, the ground lit up like the stars in the
> night sky, so we went about trying to harvest our
> fortune - each piece that fluoresced was the
> tiniest, crappiest piece of zircon imaginable, and
> needed a loupe and tweezers to pick up. None of
> the large pieces, or the gem quality pieces that
> we dug would fluoresce under SW or LW, just those
> tiny crappy pieces.
>
> The gem quality pieces that we dug ranged from
> brown, violet, pink, yellow, and colourless, but
> none of them fluoresced. I have often wondered
> "Why is it so?"
>
> Lawrie, F.G.A.
Hi Lawrie,
An interesting observation indeed. I wonder is the explanation might be along the following lines. As all gemmologists know, the larger a piece of gem rough, the rarer it is to find it internally flawless and without inclusions. Much more common to find flawless/near flawless small pieces. That being so, would it be logical to conclude that, on a random basis, the same will be true for the likely presence or absence of a fluorescent quenching accessory in the gem rough? I.e. the smaller the piece or rough, the more likely it is to be free of a quenching accessory in the crystal lattice?
Another careful sampling trial for some bright young thruster to put his name on? ;-)

16th Aug 2017 05:54 UTCLawrie Berthelsen (2)
Lawrie.
16th Aug 2017 06:24 UTCJoel Dyer
Owen, thanks for your kind comments. I've emailed you a link for downloading full-sized photos.
Sorry to prolong this thread. I have to apologize to Pavel and others for not conducting a proper, controlled experiment. But I was not carrying out an extended research project here....I don't have the equipment for that.
...
If anyone is interested, I heated the zircon so that it was red-hot or even lighter colour, using a special blow-torch with a gas mixture. First I used the flame tip, "touching" the material on/off style, so as not to crack the material all over the place in a useless and dangerous way. Then I used the blue part of the flame only.
I've heated many minerals this way, and the flame is so hot, that it melts many metals and some minerals that have a pretty high melting temperature. The highest temperature reached was likely over 1000 degrees.
...
Alfredo, many metamict samples are actually only partially metamict. So I would - in my humble non-expert view - consider the samples to be zircon, and from all the literature I've so far read, I believe this is the international way of stating things...? But please correct me if I'm wrong, I'm very thankful for any lessons learnt.
...
If the sample were very highly or completely metamict - such as Laitila zircon according to XRD and Raman - then the material doesn't naturally have a crystalline structure at all. And this kind of zircon I haven't been able to anneal by any repeated heating. Literature seems to support this, too. But gadolinite etc. seems to behave differently...
And you can see from Raman results if indeed a material is quite metamict. Raman is pretty sensitive to any changes in this way, and can most certainly give you a "half-normal" spectrum for "half" metamict zircon, eg. more information than XRD can. That is the reason for instance why the Geological Survey here has got me to run some difficult experiments with material which is otherwise "impossible".
Crystallinity and phase changes are very often monitored with Raman, even using heat/cold plates and industrial process monitoring systems with integrated Raman constantly sampling gases, solids or liquids.
Again, apologies for prolonging this thread. I just wanted to explain - as there were questions raised - what I actually did and what may be possible and not by heating & then running analyses. All this of course is already well familiar to Pavel and other analytical professionals.
Joel

16th Aug 2017 07:02 UTCAlfredo Petrov Manager
Owen: "Sidestep the linguistic trap by writing 'metamict ZrSi2O4'?"
Indeed, gentlemen, I do not disagree with you. I was merely pointing out that discussion of fluorescence or its absence in metamict material may be just a red herring when discussing fluorescence in zircon, as their properties would be expected to be different.
Joel: If you have a "partially metamict" zircon, then you either have a mixture of zircon and metamict ZrSi2O4, or you have an intermediate phase between the two, depending on how small of a nanoscale you are looking at. But "considering them to be zircon", although that may well be standard vocabulary in the literature, is at best misleading when little true zircon structure remains. I realize I'm just being the "obnoxious pedant" here, but I fear in discussions of metamict substances even the most obvious facts are often overlooked, in part because of the looseness of the traditional vocabulary.
16th Aug 2017 12:50 UTCAndrew Debnam 🌟
The metamictization of zircon: Radiation dose-dependent structural characteristics
James A Woodhead Department of Geology, Occidental College, Los Angeles, California 90041, U.S.A George R. Rossman, Leon T. Silver Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U-S.A.
click below to read the paper
Metamict Zircon structure
16th Aug 2017 13:29 UTCPavel Kartashov Manager
-------------------------------------------------------
> Sidestep the linguistic trap by writing 'metamict
> ZrSi2O4'?
Owen, in which of your sources did you found such strange formula for zircon? Or it is your own contribution to science? ;-)))
16th Aug 2017 13:35 UTCPavel Kartashov Manager
16th Aug 2017 13:42 UTCAndrew Debnam 🌟
16th Aug 2017 19:20 UTCOwen Lewis
-------------------------------------------------------
....
> Indeed, gentlemen, I do not disagree with you. I
> was merely pointing out that discussion of
> fluorescence or its absence in metamict material
> may be just a red herring when discussing
> fluorescence in zircon, as their properties would
> be expected to be different.
>
> Joel: If you have a "partially metamict" zircon,
> then you either have a mixture of zircon and
> metamict ZrSi2O4, or you have an intermediate
> phase between the two, depending on how small of a
> nanoscale you are looking at. But "considering
> them to be zircon", although that may well be
> standard vocabulary in the literature, is at best
> misleading when little true zircon structure
> remains. I realize I'm just being the "obnoxious
> pedant" here, but I fear in discussions of
> metamict substances even the most obvious facts
> are often overlooked, in part because of the
> looseness of the traditional vocabulary.
Not 'obnoxious pedantry' at all, Alfredo. Rather a timely reminder from you that it is always better to keep one's use of words as accurate as possible. Zircon is crystalline mineral. Metamict is the same chemistry (ZrSiO4) as zircon but is in the glass (disordered) state rather than the ordered crystal state of zircon. The process of metamictisation in nature occurs over some huge period of time and many samples of ZrSiO4 are found in which both the crystal and glass states are present in varying proportions according to how advanced the change to metamict is.
For those many of us without access to advanced crystallographic instruments, it is fortunate that the change in state can be observed and very crudely quantised by determining accurately the SG of samples and also the refractive indices, both of which are markedly changed but the advance in the change of material state. This enables those using these techniques to classify the material as follows:
- High type zircon. zircon in which the SG and RIs are unaffected/hardly hanged from the expected values for zircon, indicating the presence of little/no metamict material. SG = 4.70 +/- 0.03. RIs = 1.925 and 1.984 (+/-0.040).Almost all gem quality material is high type zircon.
- Low type zircon or metamict. Material in which the change to the metamict state is either fully or nearly completed. SG= 3.90 - 4.10. RI 1.78 to 1.81 and with change from doubly refractive anisotropic to singly refractive isotropic. Specimens of this low type are relatively uncommon and quite sought after as curiosities or reference samples for collections.
- Intermediate type zircon. All that by RI and SG lie between the extremes of high and low type categorisation.
Strong heating can reverse the metmict change, turning low and intermediate zircon specimens back to high type zircon.
All of which does not help us in the matter of UV fluorescence. As a general observation, the excitation of a fluorophore is unaffected by whether the fluorophore is in a glass or crystal state; or whether it is anisotropic or isotropic. The changes in state that produce a release of down-converted photon energy level occur at the sub-atomic level as described a few posts back. Accordingly, one might expect to find fluorescing metamict as often as one finds fluorescing zircon - but that is simply not the case. Thus, this remains one of life's unknowns to me. One might speculate that the eventual near extinction of radioactivity in the U/Th/Hf that causes the change to metamict also causes those exhausted accessories then to act as a fluorescence quencher. But that remains to be demonstrated.
16th Aug 2017 19:36 UTCOwen Lewis
Yes, interesting paper. Thanks for sharing.
@ Joel,
Images received. Many thanks. More by mail to follow.
@ Laurie,
Your observations are most surely interesting and, hopefully, they will be followed up. Why not do a write-up for the Journal of Gemmology?

17th Aug 2017 03:56 UTCLawrie Berthelsen (2)




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 24, 2024 03:11:54
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 24, 2024 03:11:54