Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Identity HelpElemental sulfur on magnetite?

5th Dec 2016 12:15 UTCJyrki Autio Expert
Hangaslampi
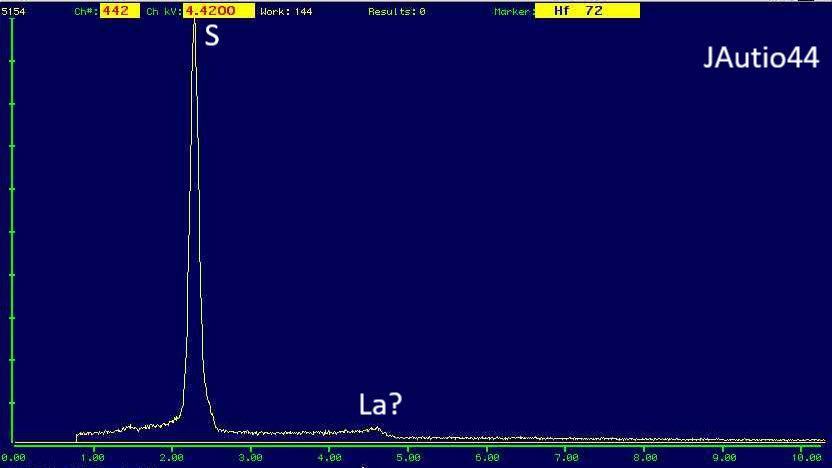
The EDS is from 0.1-0.5 mm yellow crystals. Are there other options? Some organic compound?
I suppose these are formed by a biological process. They are mainly found from spaces left from dissolved calcite or dolomite. What kind of biology can produce elemental sulfur, if it is it?
Cheers
Jyrki
5th Dec 2016 13:28 UTCReiner Mielke Expert

5th Dec 2016 13:45 UTCJyrki Autio Expert
No toluene at hand but the yellow ones are not water soluble. There is actually quite much of this finegrained yellow sand in the quarry separated, carried and deposited by the rain water.
5th Dec 2016 14:11 UTCPavel Kartashov Manager
Can't it to be some component of explosive?

5th Dec 2016 14:17 UTCJyrki Autio Expert

5th Dec 2016 14:26 UTCJyrki Autio Expert
5th Dec 2016 15:51 UTCGregg Little 🌟
Did you dissolve the carbonate out? If so what acid did you use? Could this be a reaction product or another mineral exposed after dissolving the carbonate?

5th Dec 2016 16:31 UTCJyrki Autio Expert
I did not dissolve anything. These are found from small voids left from naturally dissolved mineral which could have been calcite or dolomite.
These are found in place I did not observe any use of explosives. Only the moraine cover was removed to expose bedrock.
These were only observed in places of massive magnetite outcrops, several m2 in area.
5th Dec 2016 16:42 UTCReiner Mielke Expert
5th Dec 2016 17:44 UTCGregg Little 🌟
While prospecting in the Yukon I came across a fracture filled with an Epsom salt like mixture, probably a mixture of hydrated magnesium sulphates. Although northern Canada is cold, it is also relatively arid so, not all that surprising. I encountered surface mineralization in southern BC where gypsum (tiny ones of the rams horn habit) was growing on the surfaces inside a mine audit entrance. Another surface mineralization I have heard of are romerite and copiapite in the Spences Bridge area, BC.
Ground water acting at the till-bedrock contact could produce some interesting precipitates depending on the deposit's mineralogy.

5th Dec 2016 18:15 UTCFranz Bernhard Expert
What is the brownish grain in the upper right corner?
Maybe there were sulphides present in the S-filled vugs? Perhaps sphalerit?
The metal is gone, the S2-- was only partly oxidized and some was left behind as elemental S?
Not an unusual phenomenon.
Franz Bernhard

6th Dec 2016 02:54 UTCAlfredo Petrov Manager
6th Dec 2016 06:27 UTCChristian Auer 🌟 Expert

6th Dec 2016 12:44 UTCJyrki Autio Expert
After a little test it is obvious It burns and smells like sulfur. Freshly opened vugs seem to be half full of uniformly sized granular sulfur and when excess is poured out, what is left looks like picture in first post.
I still think the vugs are after some soluble mineral other than pyrite because there are a lot of quite unaltered pyrite cubes in magnetite and these vugs are irregular in shape.
Seems like a good subject to read more and also try to find out what the white and brown crystals are.
9th Dec 2016 16:54 UTCUwe Kolitsch Manager
11th Dec 2016 12:04 UTCJoel Dyer
It looks almost as if elongated crystals are partly frozen in "congeled" yellowish sulphur(?). There also seem to be both elongated (monoclinic??) whitish and more grain like or rounded rectangular whitish crystals. Also found a very few more reddish or pinkish crystals. So there might be 2-3 different sulphate(?) minerals involved?




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 18, 2024 13:43:56
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 18, 2024 13:43:56