Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Mineralogical ClassificationRickturnerite / IMA 2010-034

16th Nov 2010 17:29 UTCRick Turner
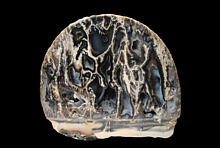
The first image (008) shows a pyramidal group of fibrous pale green rickturnerite crystals about 1cm high, on pale yellow-orange mereheadite, and calcite. This specimen is now in the National Museum of Wales, in Cardiff.
The second image (004) shows an interesting 'feather-like' aggregate of rickturnerite fibres, from my own collection. The green colour in both images is due to a small % of Cu replacing Mg. The dark spines are therefore simply thicker fibres; the mineral is white when thin.
Regards to all,
Rick
17th Nov 2010 07:03 UTCMarco E. Ciriotti Manager
Interesting phase!

17th Nov 2010 10:06 UTCSteve Rust Manager
22nd Nov 2010 02:15 UTCRobert Meyer Manager

27th Jan 2012 11:58 UTCChris Stanley Expert
M. S. Rumsey, S. V. Krivovichev, O. I. Siidra, C. A. Kirk, C. J. Stanley and J. Spratt (2012): Rickturnerite, Pb7O4(OH)Cl3, a complex new lead oxychloride mineral. Mineralogical Magazine 76, 59-73.
Rickturnerite, which has the ideal formula Pb7O4(OH)Cl3, is a new mineral from Torr
Works (Merehead) quarry, near the village of Cranmore in Somerset, United Kingdom. It occurs as
pale emerald green to grey porous aggregates of disordered interwoven minute fibrous crystals with
mereheadite, cerussite, calcite, aragonite, mimetite, hydrocerussite, ‘plumbonacrite’ and an uncharacterized
lead oxychloride, in cavities inside a manganite and pyrolusite pod. The crystals are typically
less than 5 µm wide and 200 µm long, but they can reach 40 x 100 µm in cross-section and over 1 mm
in length. The mineral is translucent with a vitreous lustre and each needle is brittle with an indistinct
cleavage, breaking with a splintery fracture. The streak is white, the Mohs hardness ~3 and the density
calculated using the empirical formula 6.886 g cm-3. Electron microprobe analyses yielded PbO 87.7,
MgO 1.79, CuO 0.14, Cl 6.62 wt.%; H2O was calculated on the basis of structural considerations as
2.27 wt.% totalling 97.02 wt.%. A charge-balanced formula, based on 12 anions, is
Pb7.16Mg0.81Cu0.03Cl3.40H4.60O8.60. Rickturnerite is orthorhombic Pnma, with a = 5.8024(6), b =
22.717(2), c = 25.879(3) A ˚ , V = 3411.2(6) A ˚ 3 and Z = 8. The diffraction pattern contains strong
reflections that define a subcell with a = 5.8034(5), b = 11.3574(9), c = 12.939(2) A ˚ , V = 852.9(6) A ˚ 3
(space group Pmm2 which is related to the real unit cell by the transformation matrix <100/020/002>),
and weak reflections that correspond to doubled b and c parameters. Since the difference between the
large and small cells is only in a number of split and low-occupancy positions in the disordered region
of the structure we provide the description of the subcell structure. The five strongest lines in the X-ray
powder diffraction pattern are as follows: 6.474, 100, (400); 3.233, 73,
(107); 2.867, 57, (705); 5.636, 44, (011); 3.112, 31, (802). The crystal structure was solved by direct
methods and refined using 1318 unique reflections to R1 = 0.063. The structure is composed of a fully
ordered part consisting of double 2+ chains of oxocentred tetrahedra extended along the
b-axis, which together with Cl_ ions form 2-dimensional blocks parallel to (001). In between these
blocks, there is a disordered region containing ordered 4_ octahedra and low-occupancy Pb
and OH sites with a slight degree of ordering; these produce the weak supercell reflections.




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 07:22:13
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 07:22:13