Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Techniques for CollectorsCleaning or Polishing Pyrite

18th Nov 2018 11:54 UTCSabrina Nicholson
I should preface this to say that I'm not a geologist, and have little knowledge in the field of mining and minerals, but am seeking professional advice.
I've been researching online to try and come up with the best method of polishing this pyrite. I read an article on here (Cleaning Pyrite) and came away pretty confused after reading at least 10 different suggestions on how to safely polish a pyrite specimen. I'm hoping there's a general consensus among you.
I don't have a garage, a hose, or particularly like the idea of messing with chemicals like Hydrochloric acid or Oxalic acid because I'm totally inexperienced.
My question is - is there something safer (besides using a brush w/ whitening toothpaste) that I can buy on Amazon or get at Home Depot to safely brighten & clean this sphere? I don't want a silvery finish. I wanted to restore the gold shine to it. Someone recommended using Simple Green or Lime Away to me. Another post that I read suggested Citric acid. I'm worried about experimenting and unintentionally ruining the sphere. Will any of the above mentioned agents work to bring back the shine? What non-toxic method (if any) will bring back the golden "display-type" shine, as shown in my photo (with the black background, below)? I've already asked the seller and he didn't seem to be sure. Many thanks.
The photo of the pyrite with the black background was the sales listing photo. The other photos (with the white background) is how the sphere sort of looks now. The photos had to be enhanced a little bit because of poor lighting.

18th Nov 2018 12:50 UTCKevin Hean
I would try rubbing the Sphere with Leather rather than Cloth because cloth will hook on all the sharp edges.
You can use a Metal Polish that contains a powder, a metal polish that you would use to polish brassware, knives and forks etc.
Some tooth pastes contain a "polishing powder", ie: a toothpaste that is not a Gel Type.
Try it out on a small section first to see the result.
I notice that your sphere is made up of many small crystals so you shouldn't have too much trouble.
All this is of course is dealing with the outer surface, to do inside the nooks and crannies you will have to do what Keith suggested and use a tooth brush.
Pyrites are known to decompose, so do yourself a favour and do some research on storage.
Good Luck....
18th Nov 2018 15:04 UTCReiner Mielke Expert
18th Nov 2018 16:17 UTCKevin Hean

18th Nov 2018 16:58 UTCAlfredo Petrov Manager
However you clean and/or polish it, I'd keep it away from human hands afterwards; handle with gloves. Natural human skin chemicals don't seem to be very good for pyrite luster.

18th Nov 2018 19:57 UTCSusan Robinson

18th Nov 2018 21:20 UTCThomas Lühr Expert
18th Nov 2018 21:31 UTCAmir C. Akhavan Expert
You can try to "dry brush" the sphere.
It sounds silly, but I've successfully cleaned a few slightly tarnished pyrites and marcasites by patiently brushing them with a dry old toothbrush - after a while they got shiny again. Give it a try before you resort to chemical treatments.
As Alfredo said, the human skin is not nice to pyrite, so I wear gloves while I clean them.

19th Nov 2018 00:15 UTCSabrina Nicholson
Do you know of a link or site where I could get it?
Thank you, Amir.

19th Nov 2018 00:24 UTCAlfredo Petrov Manager

19th Nov 2018 01:22 UTCThomas Lühr Expert
Here is the original receipe of the Waller solution from Mineralogical Record 11 (p.109) as pdf-file

19th Nov 2018 13:13 UTCSabrina Nicholson
It’s supposed to be for non-ferrous metals, so I guess it won’t work on iron pyrite.
More to the point: what else can I buy commercially in the U.S. that will actually work??

19th Nov 2018 13:15 UTCSabrina Nicholson

19th Nov 2018 14:26 UTCSabrina Nicholson

19th Nov 2018 15:10 UTCWayne Corwin
If you want to polish the finished, not rough crystals you need to get some 'wet or dry' sand paper, start with 1200 grit, use wet, and if you can find any finner 'wet or dry' paper finish with that. In just about a minute your color will come back, it doesn't take much to remove the oxide surface.
You can find the 'wet or dry' paper at almost any automotive parts shop.
No acids or chemicals!
Fast & cheep besides!

19th Nov 2018 15:28 UTCSabrina Nicholson
I could really use a method that is non abrasive and gentle, but really effective in bringing back out the shine (like you see in the sales photo with the black background).
... Isn’t there some commercially available products (in the U.S.) that are available on Amazon that I can use?
Is there some kind of polish or powder for ferrous metals like pyrite? Just need a straight answer.
I don’t have any special lapidary equipment. Just these two hands and polishing cloth.
Can someone please answer that? Thanks!
19th Nov 2018 16:11 UTCDavid Von Bargen Manager
For instance
https://www.acehardware.com/departments/automotive-rv-and-marine/auto-tools-and-maintenance/polishers-and-buffers/28983
Your biggest problem would probably be removing the polishing compound (it will get in the cracks/crevices on the sphere).
The process has to be a bit abrasive or else you won't get the tarnish off. Polishing powders will not scratch the surface because they are extremely fine grained.
19th Nov 2018 16:12 UTCKevin Hean
Here are some some powders that you may prefer.
https://www.amazon.com/s/ref=nb_sb_noss_1?url=search-alias%3Daps&field-keywords=stone+polishing+powder&rh=i%3Aaps%2Ck%3Astone+polishing+powder

19th Nov 2018 17:00 UTCSabrina Nicholson

19th Nov 2018 21:07 UTCSabrina Nicholson
19th Nov 2018 22:11 UTCKeith Compton 🌟 Manager
Try on a small section first.
If it doesn't work well enough at least you will be able to clean your silver dinner service.
Whatever cleaning product you use, I feel that the tarnish will most likely return after a short while and you will have to repeat the process.

20th Nov 2018 00:10 UTCSabrina Nicholson

21st Nov 2018 19:44 UTCThomas Lühr Expert
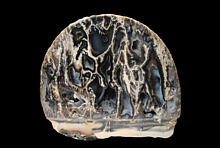
The second photo shows a specimen that already in the decomposing process (note the fissure in the lower left cube). Since the treatment (soaking for some days), a few years ago, it is unchanged and the decomposing stopped (the future will show for how long)
Thomas

21st Nov 2018 22:55 UTCSusan Robinson
I hope the readers find this information helpful,
Susan Robinson

22nd Nov 2018 02:36 UTCSabrina Nicholson
Being such a newbie to this, any ideas where I can buy this "Waller solution?" Does it make it silvery finish or will the pyrite keep its gold coloring? Is there some other name that it goes by? Would love to know the cleaning technique with the solution. I take it that you're supposed to dip it in a solution and leave it there? If so, for how long? Or, do you buff it on and then off with a leather polishing cloth? Thanks again.

12th Jan 2019 21:37 UTCTina Williams
I’m wondering if it would help this piece. Pyrite looks to be oozing out of the cracks in this one. Found in North east Tennessee on a mountain, 20 pounds about 14 inches long quartz. I love it but the pyrite is dull.
Thank you!

12th Jan 2019 22:03 UTCThomas Lühr Expert
"Iron Out" is a commercial equivalent of the Waller Solution, available in the USA.
For you it is the best to use those. As far as i know there are more products of different composition, but all called Iron Out.
The powder should be the right one. Look at the listed ingredients, to be sure: "sodium dithionite" or "sodium hydrosulfite" (different names of the same stuff) should be mentioned.
Thomas

12th Jan 2019 22:51 UTCBob Harman
I hate to respond to your postings, but from this posting and your previous postings on cleaning specimens, you seem to be way to quick on the cleaning trigger!
Other than a good washing and rinse, cleaning mineral specimens, in my opinion, should become an art. Look critically at each specimen to see what realistically (!!) can be accomplished. Can the example be realistically and meaningfully improved, both aesthetically and, if $$$ is involved, from a monetary point of view ??.
For your 20 pound example, do you think you can really clean that up using a whole lot of iron out or something else? What do you plan to do with it? Do you plan to display it on a shelf or put it in your garden? In my opinion, using a lot of iron out on that specimen will really make it look no better.
For me, be realistic, leave it as it is, putting it on display in your garden. It is a yard and garden rock. CHEERS.....BOB

12th Jan 2019 23:02 UTCDoug Daniels

12th Jan 2019 23:50 UTCBob Harman

13th Jan 2019 00:46 UTCThomas Lühr Expert
I agree with the two guys, your piece is way too large for such treatment.
You should follow to Doug's suggestion and break it apart, using hammer and chisel. With a bit luck (and skills) it breaks along the pyrite layer. Choose the best piece (maximal fist sized) and give it the treatment. The rest leave as it is.
You don't have to be worried to trigger a process of decomposing. In the contrary, the alcaline components will eliminate any sulfuric acid. The acid is product of decomposition that hastens further decomposition - a downward spiral. Iron Out prevents that. But, as Doug wrote, all the chemicals must be good washed out by a good soaking in clear water.
Thomas

13th Jan 2019 02:47 UTCTina Williams
I do not look for monetary value in any of my pieces I just love the beauty they have.
I’ve worked with super iron out powder for quite some time and I am familiar with the safety precautions and rinsing regimen that must be used with it.
I understand nothing is “oozing out” that’s just the way it appears and I was trying to describe it in case you all could not see where the Pyrite was in the picture because it’s dull.
Tina

13th Jan 2019 05:19 UTCアーロン ベリル

13th Jan 2019 19:05 UTCTina Williams

31st Mar 2019 13:59 UTCAarti Kalro
My ratio is about 1 tsp for 1.5 cups of water. You can experiment .
Good luck. !

31st Mar 2019 16:49 UTCDonald B Peck Expert
31st Mar 2019 18:38 UTCKevin Hean
As Kids, living on the Witwatersrand, there was always a Dad giving one of us some, of what they called, Iron Pyrites from a gold mine, for us to make stink bombs with by adding it to HCL.
As you would know a test to see if Lapis Lazuli contains Pyrite or is man made with little flecks of brass, a drop of HCL smells of rotten eggs on Lapis with pyrite.
I wonder what happened to Sabrina who started this Thread with her Pyrite Sphere.

31st Mar 2019 19:24 UTCAlfredo Petrov Manager

1st Apr 2019 18:55 UTCDonald B Peck Expert
1st Apr 2019 20:08 UTCKevin Hean
all we were interested in was the stink :-)
Looks like Alfredo is right as well about the H2S originating from the Lapis and not the Pyrite, and I was told this in a Gemmological lecture,
anyway you live and learn.

1st Apr 2019 21:07 UTCDoug Daniels
Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 06:30:22
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 06:30:22