Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
GeneralSalt of the earth: Halite and other evaporites
3rd Feb 2013 21:21 UTCMaggie Wilson Expert
This a 2.5 x 2.2 x 2.0 cleavage of halite from Goderich, Ontario. I got the idea to photograph it this way from someone here on Mindat - name forgotten, I'm afraid, but thanks for the tip!
Maggie
PS - the Min Rec issue by Philip Simmons is a GREAT read, and ties in with other current topics about collecting at operating mines.

3rd Feb 2013 22:19 UTCPhilip Simmons
Thanks for the kind words! I have been fortunate and blessed to be in the right place at the right time to collect the blue halite and other evaporite minerals from the Carlsbad mines. Thankfully my supervisors don't have a major problem with mineral collecting, as long as I do it on my own time. Hopefully our management will continue to maintain this enlightened philosophy concerning the preservation of our mineral heritage. I have already posted the pic below in another thread, but for the people who haven't had a chance to read the article and see some of the specimens that have been found, here is one of the best blue halites found from Carlsbad, New Mexico in the past 3-4 years. Please note that the majority of the colorless to white mineral associated with the blue halite from Carlsbad is sylvite, not halite. I have quite a few specimen photos that did not make the cut for the MR article, and I think it would be fun to post them here as time allows.
Blue halite with minor sylvite, 8.1 cm.
4th Feb 2013 05:37 UTCJake Harper Expert
Superb MR article!
I've only read it four times over since receiving it -- and I am still not done. Of course, the collecting stories are HIGHLY entertaining -- but I must say that I've become slightly obsessed with the beauty of those specimen photos as well. I really look forward to seeing more here. Thanks for a great article and your hard work!
Jake

4th Feb 2013 05:50 UTCTim Jokela Jr
Fantastic article. Amazing photos. A new world standard for halite!
Well done.

4th Feb 2013 18:32 UTCKelly Nash 🌟 Expert

4th Feb 2013 19:01 UTCPhilip Simmons
Kelly, I have been pondering how to go about what you suggest. I guess I can post a notice in the "Errors" forum and we can work from there.
Based on my mine maps both the Intrepid Potash East (Mississippi Potash mine...) and North (National Potash mine...) mines have workings in Eddy and Lea counties, although all of the mineral specimen occurences of note in the article are located in Eddy County. The Intrepid Potash North mine is the only one that has the majority of workings in Lea County.

4th Feb 2013 19:47 UTCJeremy A. Zolan
http://www.mindat.org/gallery.php?loc=15934
4th Feb 2013 22:35 UTCRonnie Van Dommelen 🌟 Manager
That was a great MR article. I enjoyed it very much. You conveyed the excitement of the finds very well and of course the specimens are amazing.
------------------------
Jeremy,
My understanding (second/third hand knowledge) is that the interesting NB borates etc were all from drill cores. I have heard in the news that there has been some preliminary work to open another potash mine nearby. Maybe there will be more drill cores!
-------------------------
My contribution to this thread will be an evaporite. This howlite only recently found its way into my collection.

5th Feb 2013 00:17 UTCRichard Gunter Expert
I really liked those photos. Blue Halite seems to be something quite unusual. The salt mines of the Windsor-Gorderich area do not have any that I know of and the only Canadian source is the Lanigan Mine. The Rocanville Potash Mine, which is in much the same strata as the Lanigan Mine, did not have any coloured Halite at all, only colourless.
Your explanation of the colouring mechanism for Halite in the MR article did not explain why it is so locallized within a deposit. The Canadian potash strata is constant over a large area and if radiation coloured one area the Halite should all be blue. My Blue Halite sample may be embedded in colourless Sylvite, though this is the first time I have seen that mentioned.
5th Feb 2013 00:58 UTCMaggie Wilson Expert
Great to see the posts. I'm glad that Philip re-posted the Carlsbad piece!
Thanks, Jeremy for directing me to the NB material, and Ronnie! Spectacular howlite - from a treacherous site and such dimensions and quality!
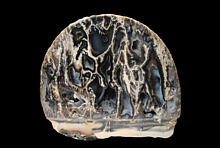
5th Feb 2013 11:19 UTCRonnie Van Dommelen 🌟 Manager

5th Feb 2013 15:24 UTCRichard Gunter Expert
That is an interesting sample. Are you sure it is a cleavage and not an embedded Halite crystal in later Halite? The blue Halite seems to need some sort of remobilization to form. The "salt pegmatites" in the Windsor Salt Mine can be quite spectacular in grain size, Halite cleavages larger than 50 cm, but not a bit of colour. Ronnie, if the university has a cleavage of blue Halite a picture would be useful. It is interesting that both the east coast examples are in salt deposits and not in potash while the New Mexico and Saskatchewan examples are in potash ore and not the accompanying Halite beds.

5th Feb 2013 16:46 UTCPhilip Simmons
Thanks for the great questions! If you notice in the article I wrote "Halite Coloration Theories" meaning that there are several theories that exist for the blue halite coloring mechanism. The major theory I presented is based on the research I did as well as observations of the environment in which the halite specimens were found.
In the occurence of blue halite at the two mines in which I work, the blue halite is always associated with colorless (or "high-grade") sylvite. I don't know much about the Canadian mines, but the vast majority of sylvite ore we mine in Carlsbad is red to orange, colored by finely divided hematite. In these areas, we never find blue halite unless there is "high-grade" present. Maybe there is something within the hematite included sylvite that inhibits the radiation effect upon surrounding halite? I have also observed many instances where two or more halite crystals are growing together in the high-grade sylvite seams, and when separated, the contact between the two halite crystals is completely colorless, while the surface areas that were in contact with the sylvite were blue to purple. This leads me to believe that the sylvite was in some way a major catalyst for the coloration of the halite. Remember, these are observations based on the occurence of halite in Carlsbad, not other world-wide occurences. I am not familiar enough with other localities to give you an explanation on coloration, especially in areas that contain no sylvite or other source of radiation.
In the article I also address another theory based on the observation you posted above. Since some of these deposits do not have a radiation source readily available, it could be possible that the color is due to the leaching of trace amounts NaBr present within the halite. Per my article (Sonnenfeld, 1995): "The phenomenon appears to be concentrated along paths of circulating brines, saturated for NaCl, that leach, brecciate and recrystallize halite and preferentially dislodge bromine ions from crystal lattices, leaving metallic sodium behind."
FYI - While any one particular coloring mechanism is not fully endorsed by all scientists, most of the research I conducted was in agreement that the metallic sodium colloids are the source of the color.
I hope this adequately answers your questions. If not, we can hash out more of these theories in detail. Like I mention above, there is more than one theory that have their strong and weak points. I chose to cover the radiation theory in the greatest amount of detail because it seems to present the most plausible explanation based on my research and real-time observations. We could also have more than one coloring mechanism, depending on the geology and type of deposit in which the halite is found.
Philip

5th Feb 2013 18:50 UTCPhilip Simmons
Here's a specimen of Aphthitalite from the major find in July 2011. The group was shot against a dark background, so you can see the transparency of the crystals. The specimen is 5.9 cm wide.
I love the color on that blue halite, Maggie!
I just added several photos of various minerals to the site, and once they get approved I will continue to post them here.

5th Feb 2013 19:39 UTCRichard Gunter Expert
I agree these are all only theories for now. One of the interesting photos you showed was of the Halite stalactites in the workings, precipitating from inflowing water. In the Rocanville Mine a similar inflow lead to the crystallization of Halite, in quite large samples. All of the posted Halite pictures from Rocanville originated from that inflow. As far as I know there are no open cavities in the Saskatchewan potash beds so all of the evaporite phases occurred as grains embedded in the coarse-grained potash.

7th Feb 2013 02:20 UTCPhilip Simmons
The specimen below is a large, completely transparent sylvite crystal that reportedly came from the famous discovery of the two enormous pockets in the PCA mine (HB Potash mine, etc.) in 1962. It is in the collection of one of my mineral collecting buddies, and was found in his uncle's or grandpa's old shed, sitting next to some old tools. The crystal is 12 x 12 cm.
7th Feb 2013 21:24 UTCStephen Rose Expert
I've had this one since 1965. It measures 10x6x4.5 cm with crystals to 1.5 cm.

7th Feb 2013 22:17 UTCPhilip Simmons
Are you sure that's sylvite and not halite? From looking at the picture, it looks like halite to me. In my experience, the sylvite from the Potash District usually has modified corners, while halite is usually strictly cubic. The easiest way to ID the specimen is to subject it to the old-fashioned taste test. :-D I do know that there have been finds many years previous to my working at the mines, so it is very possible it could be sylvite, I'm just curious.
Do you have any more specific locality data on the piece?
Since you have owned the piece for almost 50 years I'm curious as to what sort of climate you live in.
Best Regards,
Philip
7th Feb 2013 23:02 UTCStephen Rose Expert
Truth be told I am not 100% certain given the time elapsed. I got it from a shop in Northern Indiana from a guy who spent quite a bit of time in the SW in the winter and who always had some interesting Mexican things but who was mostly a 'rock shop' type dealer. I have no additional information on the location but the "Carlsbad" on the label. My guess is that I gave it a lick or two before I sprayed it with acrylic (thus the preservation), but who knows? The next time I am rooting around in that stack of boxes I will carve off a few grains of matrix for my morning egg. It will help to keep my heart going pitter-pat. :)-D
Cheers!
Steve

10th Feb 2013 04:54 UTCPhilip Simmons
15th Mar 2013 12:51 UTCSimone Citon Expert

15th Mar 2013 14:59 UTCDonald Peck
19th Mar 2017 12:22 UTCSimone Citon Expert
19th Mar 2017 14:06 UTCReiner Mielke Expert
20th Mar 2017 08:31 UTCSimone Citon Expert
has salty taste! I don't know if Gaylussite has salty taste, but is slightly soluble in water. It is absurd to think of a pseudomorph?
20th Mar 2017 12:56 UTCReiner Mielke Expert

20th Mar 2017 13:24 UTCJamison K. Brizendine 🌟 Expert
21st Mar 2017 10:00 UTCSimone Citon Expert
21st Mar 2017 11:47 UTCReiner Mielke Expert
23rd Mar 2017 11:38 UTCReiner Mielke Expert
23rd Mar 2017 13:10 UTCSimone Citon Expert
"Dear Simone;
If your sample does not react with HCl, it can not be gaylusite (Na2CO # .CaCO3.5H2O). Being a carbonate mineral, gaylusite reacts strongly with HCl and other strong acids. Gaylusite would not have a salty taste; salty taste comes from the chloride ion.
A salty taste suggests it is a chloride mineral of some sort. My best guess is Halite That has an octahedral crystal habit. I have seen octahedral Halite fairly Often in the lake minerals So THAT would not be surprising to find That You specimen is halite. Often, octahedral halite is mistaken for sulfohalite, but the Halite is normally a very clear crystal whereas Sulfohalite is opaque, yellow or brown. You can see a diagram of octahedral halite at: http://www1.iwvisp.com/tronagemclub/Diagrams.htm "
Then I took the example of the cuboctahedron found in that table, and I made a graphic design, creating a cuboctahedron deformed and modified (magnified octahedron faces). Look at the comparison with my crystal, then tell me what you think to consider this a Searles lake Halite crystal with deformed cuboctahedron habitus.
23rd Mar 2017 18:08 UTCReiner Mielke Expert

24th Mar 2017 15:24 UTCDonald B Peck Expert
Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 07:06:46
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 25, 2024 07:06:46





























Detroit Salt Company Mine, Detroit, Wayne County, Michigan, USA