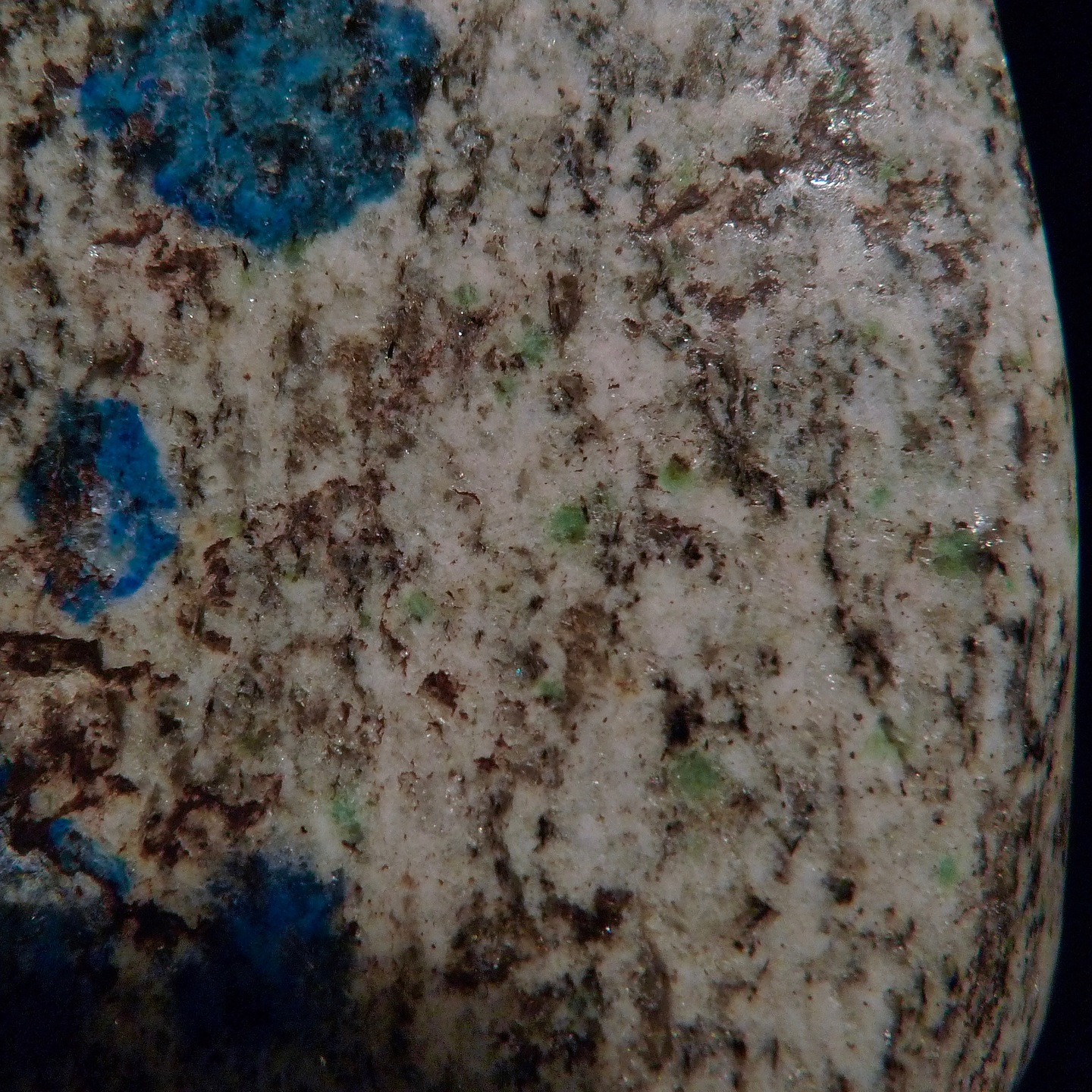Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
GeneralK2
20th Sep 2013 19:07 UTCSteve Hardinger 🌟 Expert
People who work this material tell me the blue of equal hardness with the matrix, an observation that is inconsistent with azurite. Lazulite, lazurite or blue sodalite make more sense to me.
Comments?

21st Sep 2013 01:20 UTCLeor Goldberg
21st Sep 2013 02:10 UTCSteve Hardinger 🌟 Expert

21st Sep 2013 03:14 UTCDoug Daniels
21st Sep 2013 04:27 UTCSteve Hardinger 🌟 Expert
21st Sep 2013 05:49 UTCRalph S Bottrill 🌟 Manager

21st Sep 2013 07:00 UTCDoug Daniels

21st Sep 2013 16:54 UTCDonald Peck
21st Sep 2013 17:18 UTCStephanie Martin
Check out photos of rough:
http://www.ebay.ca/itm/K2-blue-jasper-pakistan-7-78-lbs-/171121424645?pt=LH_DefaultDomain_0&hash=item27d7a1b905
http://www.ebay.ca/itm/K2-blue-jasper-pakistan-3-28-lbs-/300957958506?pt=LH_DefaultDomain_0&hash=item46127e016a
regards,
stephanie :))
21st Sep 2013 17:45 UTCRob Woodside 🌟 Manager
21st Sep 2013 18:21 UTCStephanie Martin
http://www.ebay.ca/itm/K2-blue-jasper-from-pakistan-2-10-lbs-/300957809750?pt=LH_DefaultDomain_0&hash=item46127bbc56
regards,
stephanie :-)

21st Sep 2013 18:30 UTCFranz Bernhard Expert
I had a somewhat similar specimen about 15 years ago. A friend showed gave me a slab of a fine grained, white gneiss from Hohe Tauern, Austria, with blue spots, which he believed to be lazulite. However, they were imprägnations of a copper mineral, most probable azurite (only Cu detected on grain boundaries with SEM-EDS).
"photo 3 and 4 clearly show raised surface aggregates of the blue material in host"
They appear to me to be just random elavations.
Franz Bernhard
21st Sep 2013 18:47 UTCStephanie Martin
and at least we know they are NOT mythical blue garnets ;-)

22nd Sep 2013 00:14 UTCJohn Oostenryk
Before this thread goes futher astray from Steve's analysis data inquiry... He is NOT saying anything is fake or contrived!
As he points out he isn't questioning that~. Several others(and myself) do not see fakery(ink whatever...)
I do not know the Etsy person Leor listed first, but I AM familiar with the ebay seller (Stoneclouds) that has been referenced further down this post. He is an experienced lapidarist and I will vouch for his input. I contacted him to request his commentary on cutting/crossection.
Please consider I invited him as a guest for polite comment RE: cross section appearance when he is cutting. (ie:that it is sound/not contrived)
I am hopeful he can/will post a pic or piclink to a rough edge oblique view so a cross-section is observable. That should help direct this thread back on track topically:)
Ralph~ I know you are sharp cookie and enjoy your knowledge contributions! However, today;),I would surely say goodbye to your hat-EXCEPT, you are probably safe as your comment was in regard to the "polished" piece. Unless someone buys it for destructive testing? (Do you have enemies or ornery friends watching your posts? LOL:) I trust you enjoy some humor!
Anyhoo-back on topic- Wish I could answer the original request beyond passing messages...
I do agree that hopefully there will be a reputable testing source attached to the pending XRD indicated on the Etsy page:)-
Patience and time will tell.
Best regards,
JO:)

23rd Sep 2013 02:39 UTCDoug Daniels
29th Sep 2013 23:20 UTCSteve Sorrell Expert
-------------------------------------------------------
> From the etsy pictures given, if it's not dyed or somehow irradiated I will eat my hat!
Let me know if you get a taste for those hard hats Ralph. Mine are probably outside their use by date now, and it is one way to recycle them!
:)-D
Regards
Steve

30th Sep 2013 01:28 UTCHenry Barwood
30th Sep 2013 04:38 UTCStephanie Martin
The seller of the rough pieces above indicates on his website that the material was tested, the matrix was identified as quartz, albite and microcline (black mineral not identified). No surprise there. Further the blue material was as follows taken verbatim from the website:
"The LAB has Identified the Blue Orbs as AZURITE, with Manganese, Titanium, Strontium, and Chromium, as Secondary Minerals of Concentration."
I inquired about the type of testing and if he could post it here, he replied he didn't have a copy of the results from the lab in England. He didn't really have time to discuss as he was leaving for a field/road trip and would not be back until Nov. So no luck pinning down the scientific data just yet.
Another seller offers that the material was tested by a certified gemologist (a gemologist!) and that the azurite has not oxidized and turned black due to the cool dry climate where it was discovered.
So looks like we have to wait a little longer to get published data.
regards.
stephanie :-)
30th Sep 2013 08:49 UTCHarjo Neutkens Manager
????
30th Sep 2013 14:14 UTCStephanie Martin
regards,
Stephanie :-)
1st Oct 2013 01:07 UTCOwen Lewis
1st Oct 2013 05:28 UTCStephanie Martin

1st Oct 2013 20:49 UTCMichael Hatskel
One of the possibilities is that it is an amazonite-bearing granite and the blue grains are blue amazonite.
Such granites are known in Mongolia, China, and former Soviet Central Asia, in particular.
1st Oct 2013 21:08 UTCSteve Hardinger 🌟 Expert
1st Oct 2013 21:11 UTCSteve Hardinger 🌟 Expert

1st Oct 2013 22:28 UTCMichael Hatskel
I realize that it may be hard to believe that such blue, "green-free" amazonite can exist, but it does. I wish I had a picture to post as a proof, but I don't have a specimen in my collection - I just remember seeing them. Cool stuff!
Maybe Pavel Kartashov or someone else has one from Russia or Mongolia.
If you look at the ebay pics from the links provided in Stephanie's post of Sept 21, you may agree that the blue grains look like a rock-forming mineral grains, rather than the alteration product or the accessory mineral. The blue grains sitting on the edges of the rock pieces are especially interesting: you can see that they are translucent.
1st Oct 2013 23:02 UTCHarjo Neutkens Manager
Looks dyed to me, but I can be wrong (and have been in many cases...)
1st Oct 2013 23:48 UTCSteve Hardinger 🌟 Expert

1st Oct 2013 23:53 UTCMichael Beck

1st Oct 2013 23:58 UTCHenry Barwood
2nd Oct 2013 00:11 UTCSteve Hardinger 🌟 Expert

2nd Oct 2013 00:17 UTCMichael Beck
2nd Oct 2013 00:26 UTCStephanie Martin
regards,
Stephanie :-)
2nd Oct 2013 01:08 UTCIbrahim Jameel Expert
2nd Oct 2013 01:27 UTCDean Allum Expert
http://www.mindat.org/photo-354783.html
It is surprising that there are no signs of malachite.

3rd Oct 2013 07:04 UTCStephanie Martin
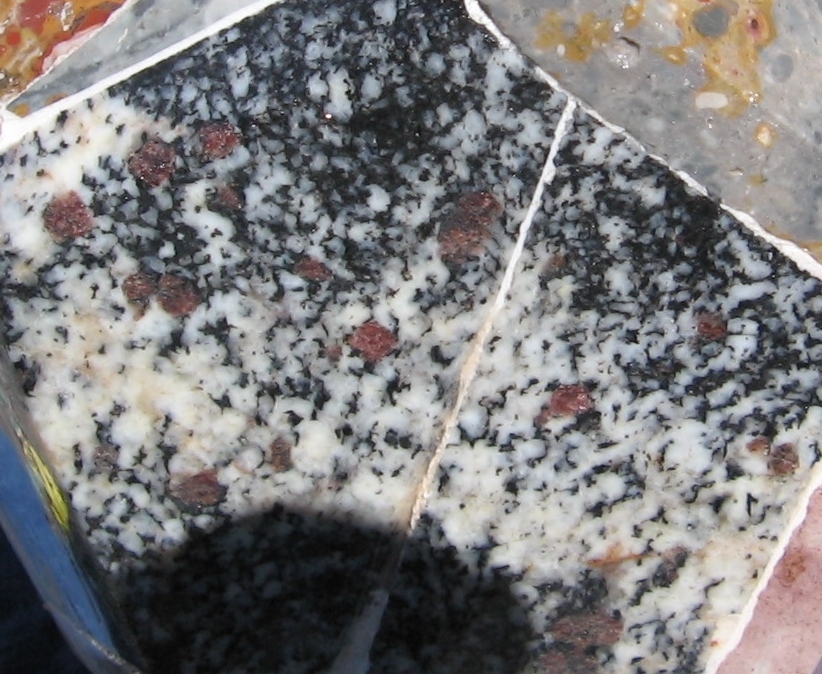
Yes I believe there are signs of malachite but it depends on the piece. There are some green edges on some of Michaels's slabs above. One of my pieces appears to be a weathered portion that shows green patches which I assumed were malachite. I finally had a chance to snap a couple photos. I haven't looked at them under the scope but I think I see some small sprays in the close-up.
overall slab approx. 9.0 x 8.0 cm
FOV aaprox 1.5 cm
regards,
stephanie :-)
3rd Oct 2013 08:12 UTCHarjo Neutkens Manager

3rd Oct 2013 08:14 UTCMichael Beck

3rd Oct 2013 08:32 UTCMichael Beck

3rd Oct 2013 16:33 UTCDonald Peck
3rd Oct 2013 16:35 UTCRob Woodside 🌟 Manager

3rd Oct 2013 16:50 UTCMichael Hatskel
3rd Oct 2013 17:31 UTCStephanie Martin
regards,
stephanie :))
3rd Oct 2013 23:19 UTCSteve Hardinger 🌟 Expert

4th Oct 2013 01:02 UTCHenry Barwood
4th Oct 2013 01:39 UTCSteve Hardinger 🌟 Expert

4th Oct 2013 02:05 UTCMichael Beck

4th Oct 2013 03:16 UTCAlfredo Petrov Manager
I also have some granite from Antarctica with 3-dimensional copper staining - Either the granite is more porous than it looks, or the penetrative staining power of tiny amounts of secondary copper minerals is quite astonishing. Naturally, with staining like this, a lapidarist is not going to notice any hardness difference between the stained and unstained "feldspar".
So I fear none of the possibilities mentioned so far in this thread has been scientifically eliminated yet, and analyses will be required.
4th Oct 2013 03:53 UTCSteve Hardinger 🌟 Expert

4th Oct 2013 04:17 UTCAlfredo Petrov Manager
4th Oct 2013 06:17 UTCStephanie Martin
here's a few more photos:
regards,
Stephanie

4th Oct 2013 13:12 UTCHenry Barwood
If you have a small piece you can send me, I will prepare a thin section and let you know what type of rock this is and make an attempt at identifying the blue material.
4th Oct 2013 14:39 UTCSteve Hardinger 🌟 Expert

5th Oct 2013 16:18 UTCBill Cordua 🌟 Manager
5th Oct 2013 17:41 UTCSteve Hardinger 🌟 Expert

5th Oct 2013 19:12 UTCBill Cordua 🌟 Manager
If you can see the original mineral textures beneath the blue - or whatever color - that's enough for me. For analytical proof, X-ray the blue stained feldspar and show it's still feldspar. - Bill
5th Oct 2013 19:34 UTCSteve Hardinger 🌟 Expert

5th Oct 2013 20:30 UTCMichael Hatskel
I would assume that the food colorant be extracted from the rock using the same solvent that was used in applying it (water or alcohol). Do you observe that?

5th Oct 2013 21:23 UTCBill Cordua 🌟 Manager

5th Oct 2013 21:47 UTCCA

6th Oct 2013 01:45 UTCAlfredo Petrov Manager
If it is just organic dye, could one test it with bleach?

6th Oct 2013 02:09 UTCHenry Barwood

6th Oct 2013 03:02 UTCJohn Oostenryk
Uh - Bill C?
Did you actually check the pics here and on Ebay and elsewhere and REALLY look at them??
If faked, how do they get the spherical blue spots inside massive rough? On second thought- don't bother answering that, it is a waste of time.
I was amused by your attempting to fake a blue stain. Go ahead- see what you can create:)
However, until you have actually physically investigated the true material you have visually snap judged, you are doing science and yourself a major disservice declaring fraud via the failed reasoning process applied:(
Looking at something in a picture, then deciding to take a similar colored/textured rock (you don't know what the host material REALLY is either.)*
Next taking your closest convenient blue colorant. (Why not ink or paint-they are blue too?!)
Then imitating bad art and finally declaring the dissimilar result to be proof that your initial baseless claim is correct is just compunding bad to worse to worst...
Gadzooks! When you really look at your comparison, you have steaks and oranges, or somesuch, both food but that is it.
Nuff said, I will shut up now~
I DO look forward to lab results from one of the generous offers presented by our fellow members:) It IS mineralization, likely something simple, We should be so lucky if it is complex. Which = "even more interesting!"~lol)
*Thank you Henry for angling for thin section ID- Heck Yeah! Having experience doing that is awesome, you rock!:) I wish my petrology course hadn't been so short. Course, it was sorta painful too, so after wasn't all bad~LOL.
6th Oct 2013 04:40 UTCStephanie Martin
Sorry I couldn't respond sooner, I will send you a PM.
regards,
stephanie :-)

6th Oct 2013 04:46 UTCCA
Regards,
Chris

6th Oct 2013 11:16 UTCMichael Beck

6th Oct 2013 14:26 UTCBill Cordua 🌟 Manager
Yes, I have looked at the photos, and they don't convince me. Next time I get to a rock show and I will be quite curious to see some " in person", as clearly a better alternative to seeing photos. I also look forward to thin section and other creditable analyses by someone like Henry. However I did prove that some granitic rock is porous enough to take a stain in 3-D using a common, readily accesible substance. I never said I had proved the K-2 material is a fake, merely voiced my suspicion based on my observations. As you all know there are a lot of modified, misrepresented and faked materials on the market today, so it makes sense to me to be skeptical. Caveat emptor.
6th Oct 2013 19:06 UTCRob Woodside 🌟 Manager

6th Oct 2013 19:52 UTCAmanda Hawkins
-------------------------------------------------------
Interesting
> that the attribute for azurite (the first ID) is
> "physical awareness"...we want to be physically
> aware of what it is, mayhaps?
lost me
6th Oct 2013 23:38 UTCHarjo Neutkens Manager
At the moment I'm putting my money on it being natural, despite my previous doubts. I can't wait to see the analysis results.
p.s. Amanda, I think nobody here cares about which moment you lost it.

7th Oct 2013 00:02 UTCDoug Daniels

7th Oct 2013 09:08 UTCAmanda Hawkins

7th Oct 2013 15:18 UTCBill Cordua 🌟 Manager
Also, I noticed some vendors linking the K2 name with jasper! Ouch! Whatever this turns out to be - it is not jasper.

7th Oct 2013 15:30 UTCChester S. Lemanski, Jr.
7th Oct 2013 17:46 UTCStephanie Martin
Chet, I can assure you that they are not just on the surface, although in the photo it may appear that way.
regards,
stephanie :-)

14th Oct 2013 04:25 UTCHenry Barwood
Material appears to be a quartz-mica (muscovite) schist, rather than a granite. There are grains of "biotite" throughout. Likely some feldspar also.Won't be able to tell more until I can examine under a petrographic scope.
The green mineral is in flat flakes that do not show a pronounced cleavage, nor fibrosity as would be expected with malachite. Definitely occurs along fracture surfaces and is not dispersed within the sample.
The blue grains are translucent and contain some darker areas of blue internally. Could be a stain, but broken grains show a uniform blue color instead of a darker rim. The grains appear to be quartz and muscovite with a strange colorant.
The material does not appear to be a product of dispersed copper mineralization, but could be. For one thing, there is no accompanying Fe staining from something like chalcopyrite. There is also no evidence of significant weathering or hydrothermal alteration of the rock.
All in all an oddity.
Hope to have more information in a week or so.
14th Oct 2013 04:54 UTCSteve Hardinger 🌟 Expert
I have received a few samples of K2 for analysis, but if you'd like to take the lead, by all means please do so. You've more experience along these lines than I.
My samples do have some of the earthy, powdery green material. This material dissolves with effervesence in dilute HCl at room temperature, so I'm guessing it might actually be malachite.

14th Oct 2013 12:25 UTCHenry Barwood
All I'm doing is some petrographic work. Please proceed with your analyses. Since this does appear to be some copper mineralization, I have to tell you that it is weird.
14th Oct 2013 14:53 UTCSteve Hardinger 🌟 Expert

15th Oct 2013 04:28 UTCTim Jokela Jr
Thanks for your analytical work, boys.
Anybody care to recommend a good source for this material, I'd like a few pounds.
15th Oct 2013 05:53 UTCSteve Hardinger 🌟 Expert

17th Oct 2013 04:16 UTCMichael Hatskel
"Raindow calsilica"... What is that? http://www.ebay.com/itm/Rainbow-calsilica-lapidary-slab-85-grams-for-cabochons-0728E-/141053193775?pt=LH_DefaultDomain_0&hash=item20d76cee2f
For the K2 material I had at least some assumptions, but not for this one...
Michael
17th Oct 2013 04:50 UTCStephanie Martin
It is definitely man made. I believe it is some type of glass with opaque colouring in banded layers. I have a number of pieces but don't think it is worth testing since I know it's not natural. But it sure is colourful. Part of my fakes collection (sub-collection?).
regards,
stephanie :-)

17th Oct 2013 14:18 UTCMichael Hatskel
The border lines between the bands look so sharp, and that it not easy to achieve in glasses.
17th Oct 2013 17:43 UTCReiner Mielke Expert
17th Oct 2013 19:55 UTCStephanie Martin
Michael, there are other things mixed in with the glass, supposedly one of them is calcite (calcium?), suggesting where they got the name "cal-silica". Maybe this makes the layers adhere better?
edit - in the ebay listing they indicate that this was confirmed to be a natural material - however it does not indicate who tested it and how (meaning lab or qualified person) , and I have never seen photos of this material in situ, or even boulder chunks for that matter (only slabs and cabs).
regards,
stephanie :))

18th Oct 2013 22:12 UTCCA
Regards,
Chris

18th Oct 2013 23:43 UTCHenry Barwood
19th Oct 2013 15:39 UTCSteve Hardinger 🌟 Expert
I have some guesses as to the nature of the blue stuff, but I'm going to hold them until my own analytical work is done.

19th Oct 2013 16:09 UTCHenry Barwood

19th Oct 2013 16:43 UTCDonald Peck
19th Oct 2013 18:07 UTCStephanie Martin
Glad your results were such as mine. I forgot to mention that the HCL did turn a more yellow. I only let mine sit for about 5 minutes as I was looking for a carbonate reaction, which was not easily detected. After I drained the acid and let the grains air dry they were looking more bleached out.
regards,
stephanie

19th Oct 2013 18:55 UTCDoug Daniels

20th Oct 2013 03:57 UTCTim Jokela Jr
Not to be confused with the old and now hard to find and quite valuable "fordite", produced by the overspray from big auto plant paint operations. The latter isn't just from Detroit; some has been saved from Toronto area plants, and presumably others as well.
20th Oct 2013 04:34 UTCStephanie Martin
regards,
stephanie :-)
20th Oct 2013 05:51 UTCSteve Hardinger 🌟 Expert
20th Oct 2013 06:07 UTCStephanie Martin

20th Oct 2013 07:00 UTCDoug Daniels

20th Oct 2013 08:54 UTCAlfredo Petrov Manager
20th Oct 2013 12:51 UTCReiner Mielke Expert
20th Oct 2013 16:30 UTCSteve Hardinger 🌟 Expert

20th Oct 2013 16:58 UTCDonald Peck

20th Oct 2013 17:00 UTCHenry Barwood
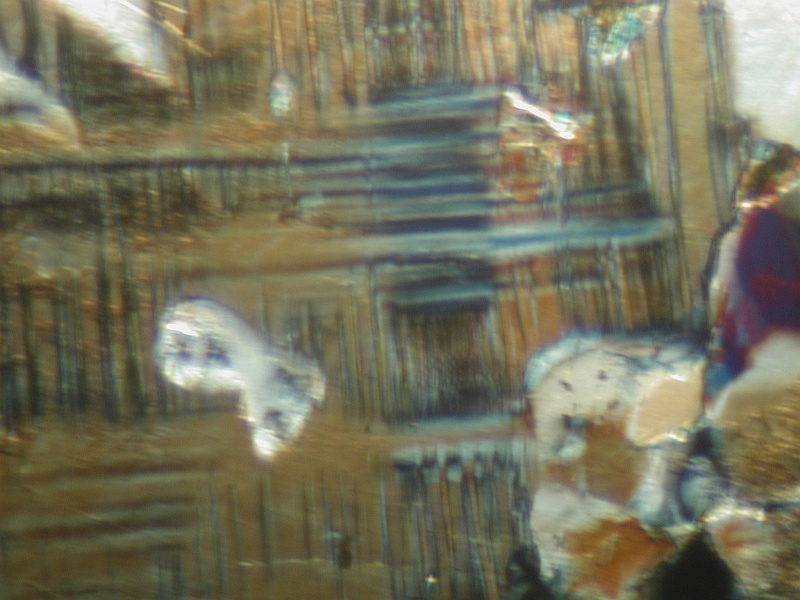
I took about 5 minutes and looked at the thin sections this morning. The coloration is bright blue, irregular grains embedded in a matrix of microcline, quartz, biotite muscovite, and perhaps a plagioclase feldspar (not twinned significantly). The grains have a high birefringence and it is hard to tell extinction patterns. Could be azurite. I'll have more when I can sit down and really examine them. I'll post some images as I have time. The basic rock is apparently a granite gneiss or possibly a grano-diorite that has been metamorphosed. I did see some sausseritization of the feldspars.
20th Oct 2013 18:28 UTCRob Woodside 🌟 Manager
"The replacement, esp. of plagioclase in basalts and gabbros, by a fine-grained aggregate of zoisite, epidote, albite, calcite, sericite, and zeolites. It is a metamorphic or deuteric process and is frequently accompanied by chloritization of the ferromagnesian minerals.
I knew the glossary would be useful!!!

20th Oct 2013 18:38 UTCHenry Barwood
As I said previously: Spending may more time on this crud than is warranted.
20th Oct 2013 18:54 UTCRob Woodside 🌟 Manager

21st Oct 2013 05:15 UTCMichael Beck
here is the lab report I was given from the gentleman I am cutting the material for. hope it is helpful. thank you to everyone out there who is making an effort to figure this out. I am very grateful for the time and effort you are all expending.

21st Oct 2013 13:55 UTCHenry Barwood
Some problems I have with this analysis:
Assuming oxides, and not elements, were reported, it sums to 116.55%. If indeed elements were reported, the sum would be even more outlandish.
I'm assuming that the acid digestion removed silicon as SiF4 during digestion, so this reports the composition of the non-volatile residue.
Looking at the thin section, you can account for a modest amount of iron from the biotite, but the level reported is way out of line with what I'm seeing.
The Al, Ca and Ba levels are really high. It is possible that you are seeing a high alumina mineral, and barian orthoclase (or even celsian or hyalophane) would not be unreasonable.Other than that, the analysis makes no sense for this type of rock, at least based on the thin section.
Something is definitely out of whack here.
21st Oct 2013 15:08 UTCSteve Hardinger 🌟 Expert

21st Oct 2013 16:48 UTCDoug Daniels

21st Oct 2013 20:22 UTCJim Robison

21st Oct 2013 20:45 UTCHenry Barwood
I so seldom post anything, I've forgotten how to make it active in the post.
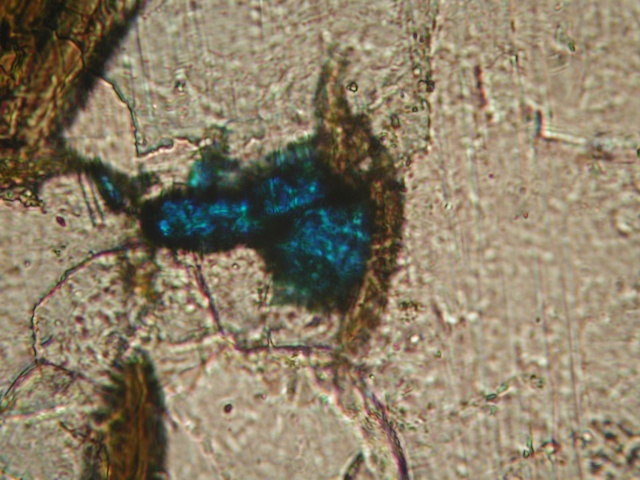
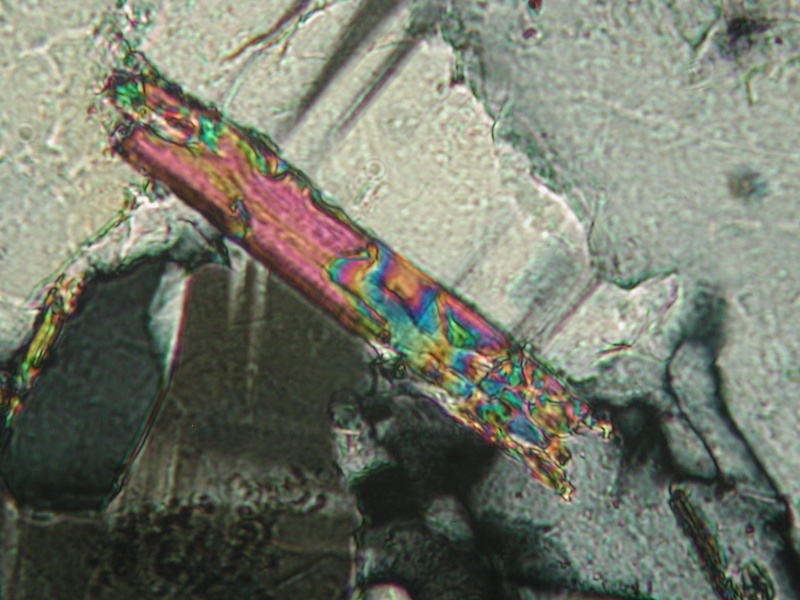
This is a typical irregular grain at 100X. Surrounded by quartz and orthoclase ? Does not look like azurite to me.

21st Oct 2013 21:47 UTCEvan Johnson
21st Oct 2013 22:48 UTCSteve Hardinger 🌟 Expert
As for the matrix question, it's not porcelain because the matrix is heterogeneous: It contains white areas (feldspar?) flecked at random with small silvery to gray to black specks (muscovite or biotite?).

21st Oct 2013 22:54 UTCBill Cordua 🌟 Manager
21st Oct 2013 22:56 UTCSteve Hardinger 🌟 Expert

21st Oct 2013 23:40 UTCHenry Barwood
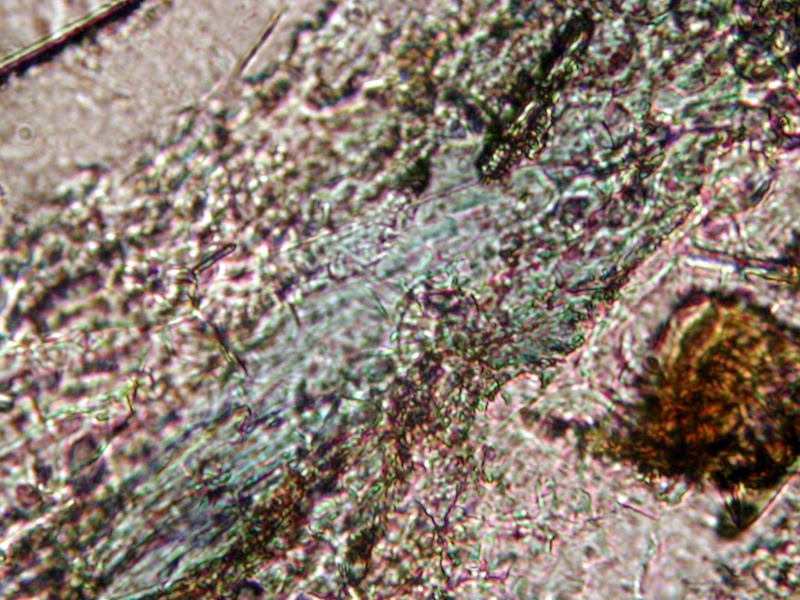
Here is a field of the grains at 40X
22nd Oct 2013 04:21 UTCSteve Hardinger 🌟 Expert

22nd Oct 2013 04:38 UTCHenry Barwood
22nd Oct 2013 06:04 UTCDon Windeler
Michael Beck passed along a lab report in a message above:
---- Scank2labreport.pdf
I realize it was probably written as
---- Scan k2 lab report.pdf
...but considering the universal disdain in which the results seem to be held, is it wrong that the first few times my brain saw it as
---- Scank 2 lab report.pdf ? :-D
Eagerly awaiting the results of all this sleuthing, as I've seen a little as well and wondered what the heck was going on. Part of me has wondered a bit about the blue datolites found at the Centennial Mine in the Keweenaw (which I vaguely seem to recall were aurichalcite possibly related to weathering of copper) or my own idle speculations about the larimar of the DR, but this stuff just looks so weirdly regular it makes you wonder what's happening!
Cheers,
D.
22nd Oct 2013 12:19 UTCStephanie Martin
For example, with those TV advisories that say: "Contains violence, viewer discretion is advised".
my brain scrambles it and becomes "Viewer desertion is advised".
regards,
stephanie :-)

22nd Oct 2013 12:58 UTCHenry Barwood
22nd Oct 2013 15:19 UTCSteve Hardinger 🌟 Expert

22nd Oct 2013 17:26 UTCChris Araujo

22nd Oct 2013 21:38 UTCMichael Beck

22nd Oct 2013 21:48 UTCHenry Barwood

23rd Oct 2013 19:31 UTCChris

31st Oct 2013 14:51 UTCChris
Thanks,
Chris

1st Nov 2013 01:44 UTCHenry Barwood
1st Nov 2013 13:29 UTCSteve Hardinger 🌟 Expert

1st Nov 2013 14:03 UTCHenry Barwood
hbarwood@troycable.net

4th Nov 2013 00:32 UTCHenry Barwood
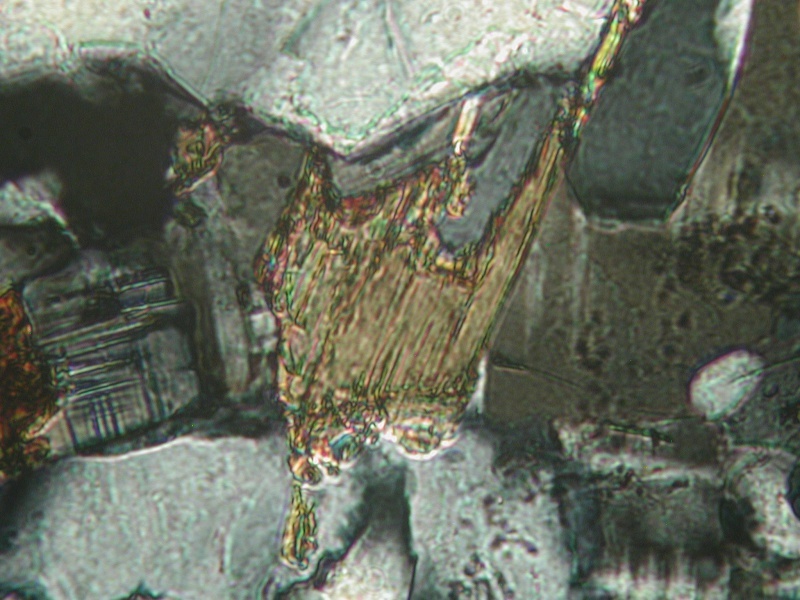
Biotite in K-2 Cross polarized light (XPL), showing high birefringence of the biotite
Pale green chlorite, PPL. There is not much of this in K-2. These may also be what appear to be flakes that look sort of like malachite on the fracture faces.

4th Nov 2013 00:37 UTCHenry Barwood
Perthite in K-2 showing typical albite twinning (XPL).
Muscovite in K-2 showing typical high birefringence (XPL)

4th Nov 2013 00:41 UTCHenry Barwood
4th Nov 2013 05:26 UTCSteve Hardinger 🌟 Expert
5th Nov 2013 04:35 UTCSteve Hardinger 🌟 Expert

8th Nov 2013 00:21 UTCChris Araujo
It's apparent that there appears to be private speculation, but nothing for public consumption.

8th Nov 2013 00:47 UTCHenry Barwood
I will not have access to EDS or XRD until later in the year. At that time I may be able to shed some light on what they are, but since this is not a research topic of mine, I've just been chipping away at the edges. Problem is that there is no context for the samples. We have no clue as to their exact origin or the geology of that origin. Such information would be of great use in interpreting what the stuff is. All I know at this point is that it is a granitoid rock with oddly distributed blue spots that have been identified previously as a copper carbonate. The presence of sausserite in the feldspars indicates that some hydrothermal alteration has taken place. It is possible that some copper minerals have developed from a copper bearing feldspar, possibly spherulitic in nature. This, of course, is entirely speculation on my part.
8th Nov 2013 14:40 UTCSteve Hardinger 🌟 Expert

8th Nov 2013 15:48 UTCLeor Goldberg

8th Nov 2013 19:51 UTCLeor Goldberg
17th Nov 2013 05:51 UTCStephanie Martin
Phosphorus is not one of the minerals listed in the "analysis" that Michael kindly posted. The aluminum content is listed and is a fair amount.
The recent request in the forum for the confirmation of the hardness of trolleite got me to thinking. Typically I wouldn't think of trolleite forming spheres, but then I ran across this material:
http://www.ebay.ca/itm/GemsVillage-64-Ct-NEW-MATERIAL-SUPER-RARE-NATURAL-TROLLEITE-FROM-BRAZIL-/171155048657?pt=LH_DefaultDomain_0&hash=item27d9a2c8d1
Now I realize this is included in quartz, but I couldn't help but notice a striking similarity. The darker blues could/would suggest a lazulite or scorazlite component. Just thinking out loud.
now that I got that out, back to the drumroll...
regards,
Stephanie :-)

17th Nov 2013 14:25 UTCHenry Barwood
17th Nov 2013 16:26 UTCSteve Hardinger 🌟 Expert
The material has at least three distinct phases, and here's what's known so far.
White phase: A feldspar. Dark spots: A mica, probably biotite. Blue spots: Contain copper, but in what form...? Well, therein lies the difficulty.
17th Nov 2013 17:57 UTCStephanie Martin
I know it didn't make sense... but then a lot of this doesn't. I know the analysis "report" was not that helpful. I was just throwing it out there, outside the box.
regards,
stephanie :-)

7th Dec 2013 18:52 UTCChris Araujo

7th Dec 2013 21:07 UTCHenry Barwood
I've got it on my list of to-do's, but will have to wait for time on an SEM/EDS analyzer. Since this is not my primary research area, it has to wait until I have time on the instrument. As you say, most people could care less what the pretty rock really is.
BTW, there are services where you can send a grain and get a qualitative analysis using EDAX for around $10.
7th Dec 2013 22:38 UTCReiner Mielke Expert
Wow 8 pages and still no answer! If you send me a small grain ( 0.1-0.5mm) of the blue stuff taped to a Christmas card I will do some chemical tests for you. However I will only be able to tell you if it is a carbonate or not and if it contains copper. I would suggest also sending a small grain ( about 0.5mm or less) to Kerry Day for EDS. He will be able to tell you if there is more than just copper in it. The cost is only $10 for EDS see: http://kaygeedeeminerals.com/sem-eds_service

8th Dec 2013 04:53 UTCFranz Bernhard Expert
"K2" is a granitic/syenitic, slightly hydrothermally altered gneiss. The blue balls are the same stuff as the matrix, except that they contain additionally a small amount (< 5%) of very small (< 50 Mikrometer) grains of azurite, giving it the blue color. Correct?
Franz Bernhard
8th Dec 2013 12:52 UTCReiner Mielke Expert
I don't think that azurite has been positively established ( unless you see something in this thread I missed). There is doubt as to whether the blue is even a carbonate or if it even contains copper? It seems people have started to work on the problem but have never completed the work for whatever reason. However I tend to agree with your conclusions but that still has to be proven.
8th Dec 2013 16:33 UTCSteve Hardinger 🌟 Expert
8th Dec 2013 16:54 UTCReiner Mielke Expert
Thanks for clarifying that. Did the microprobe show anything else present such as Al, Si etc.? How about the anion? Was there any S present? The acid tests did not seem to be conclusive with respect to carbonate being present? Looks like XRD is needed which should be able to Id a mixture.

8th Dec 2013 16:57 UTCHenry Barwood

8th Dec 2013 22:40 UTCDoug Daniels
8th Dec 2013 23:39 UTCReiner Mielke Expert
9th Dec 2013 05:26 UTCRock Currier Expert
9th Dec 2013 14:33 UTCReiner Mielke Expert

10th Dec 2013 02:11 UTCJamie Cheshire
James
10th Dec 2013 12:28 UTCRock Currier Expert
10th Dec 2013 14:18 UTCReiner Mielke Expert
10th Dec 2013 19:59 UTCRock Currier Expert
11th Dec 2013 01:26 UTCReiner Mielke Expert
18th Dec 2013 21:07 UTCSteve Hardinger 🌟 Expert
I've just heard some K2 analysis news from John Attard. He was able to isolate a grain of blue material and run EDX. The EDX confirms this material to be azurite. The EDX spectrum is attached.
Thanks, John, for your work on this material.
Now that its composition is confirmed, it's time to debate it's origin.

18th Dec 2013 21:18 UTCHenry Barwood
18th Dec 2013 21:45 UTCSteve Hardinger 🌟 Expert
And now I've looking for more specific locality information. Does anyone have a precise locality for the K2 deposit, or maybe can put me in touch with somone who might have this information?
19th Dec 2013 01:07 UTCReiner Mielke Expert
19th Dec 2013 05:27 UTCStephanie Martin
cheers,
stephanie :-)
19th Dec 2013 05:53 UTCSteve Hardinger 🌟 Expert

19th Dec 2013 09:19 UTCJohn Oostenryk
Just teasin of course, lol...
That is great that Mr. Attard was able to get you(and everyone) a definitive answer. Cool!
As to the second portion: Locale!
~ Back on page 2- Ibrahim Jameel of Khyber Minerals said he had seen a dealer with a big chunk in Skardu, Pakistan, in 2006... I'd suggest dropping him a PM or an email via his web shop. Possibly he could direct you to that contact?
~JO:)
19th Dec 2013 17:47 UTCReiner Mielke Expert

20th Dec 2013 08:53 UTCMichael Beck

16th Jan 2014 06:44 UTCPaul Gomez
-------------------------------------------------------
> I think it is natural, polishing it brings out the
> intense colour, they do resemble azurite suns but
> until testing confirms it is just a guess,
> although scorzalite does seem to be a better fit.
>
>
> Check out photos of rough:
>
> http://www.ebay.ca/itm/K2-blue-jasper-pakistan-7-7
> 8-lbs-/171121424645?pt=LH_DefaultDomain_0&hash=ite
> m27d7a1b905
>
> http://www.ebay.ca/itm/K2-blue-jasper-pakistan-3-2
> 8-lbs-/300957958506?pt=LH_DefaultDomain_0&hash=ite
> m46127e016a
>
> regards,
> stephanie :))

16th Jan 2014 07:08 UTCPaul Gomez

16th Jan 2014 14:42 UTCBill Cordua 🌟 Manager

20th Jan 2014 15:01 UTCDan Costian
Dr. James Carter from UTD confirmed that as a fact.

20th Jan 2014 15:04 UTCDan Costian
Dan
20th Jan 2014 16:45 UTCReiner Mielke Expert
20th Jan 2014 16:53 UTCReiner Mielke Expert

20th Jan 2014 17:09 UTCAlfredo Petrov Manager

20th Jan 2014 17:13 UTCDan Costian
Please see my http://www.mindat.org/photo-587533.html mentioned by Reiner.
My guess is that John Attard worked on a different stuff also called "K-2".
20th Jan 2014 18:04 UTCSteve Hardinger 🌟 Expert
The blue material is microcrystalline at best; tiny blue specks localized within the matrix so that they appear to be sphere or crystals on cursory inspection.
When immersed in dilute HCl, gas is released from the specimen. Very tiny amounts from across the entire surface (as liquid displaces air pockets in the matrix) but visibly more vigorously from the blue pods. Within a few minutes, all the outgassing stops and the blue pods have been leached.
Sodalite, lazurite, and related suggestions wouldn't give this extra effervescence. Nor would they be consistent with the XRD data.
Of all the guesses made so far as to the mineralogical identity of the blue pods, the only one consistent with all the observations -- and the only one consistent with the best test applied so far (XRD) is azurite.
Azurite has been questioned because the genesis seems wrong. I agree it's weird, but I seem to recall something about the shaving equipment of a certain Occam, and accepting unusual conclusions.

20th Jan 2014 18:40 UTCDan Costian
My blue stuff on matrix (which I bought as K-2) does not fizz at all with HCl 10%. Maybe we are talking about different materials, I cannot explain otherwise the confusion.
Here is a photo of my K-2 (which I am firmly convinced it's sodalite - besides non fizzing, the white streak and hardness etc confirming it) and below a photo of real azurite (with some red spots of cuprite) concretions on matrix.
Dan

20th Jan 2014 19:11 UTCBill Cordua 🌟 Manager
20th Jan 2014 23:40 UTCJosé Zendrera 🌟 Manager
The azurite hypothesis has an analisys to support itself.
Has the sodalite hypothesis any homologable evidence?
I think that analitic results are more reliable than a naked-eye examination, even from expert people.
In other hand, there is an important issue which has yet pointed by Bill: sodalite is exclusively formed in alkaline rocks, never in acid rocks as granitoids. Conversely, azurite is not rarely found in granites as secondary mineral.
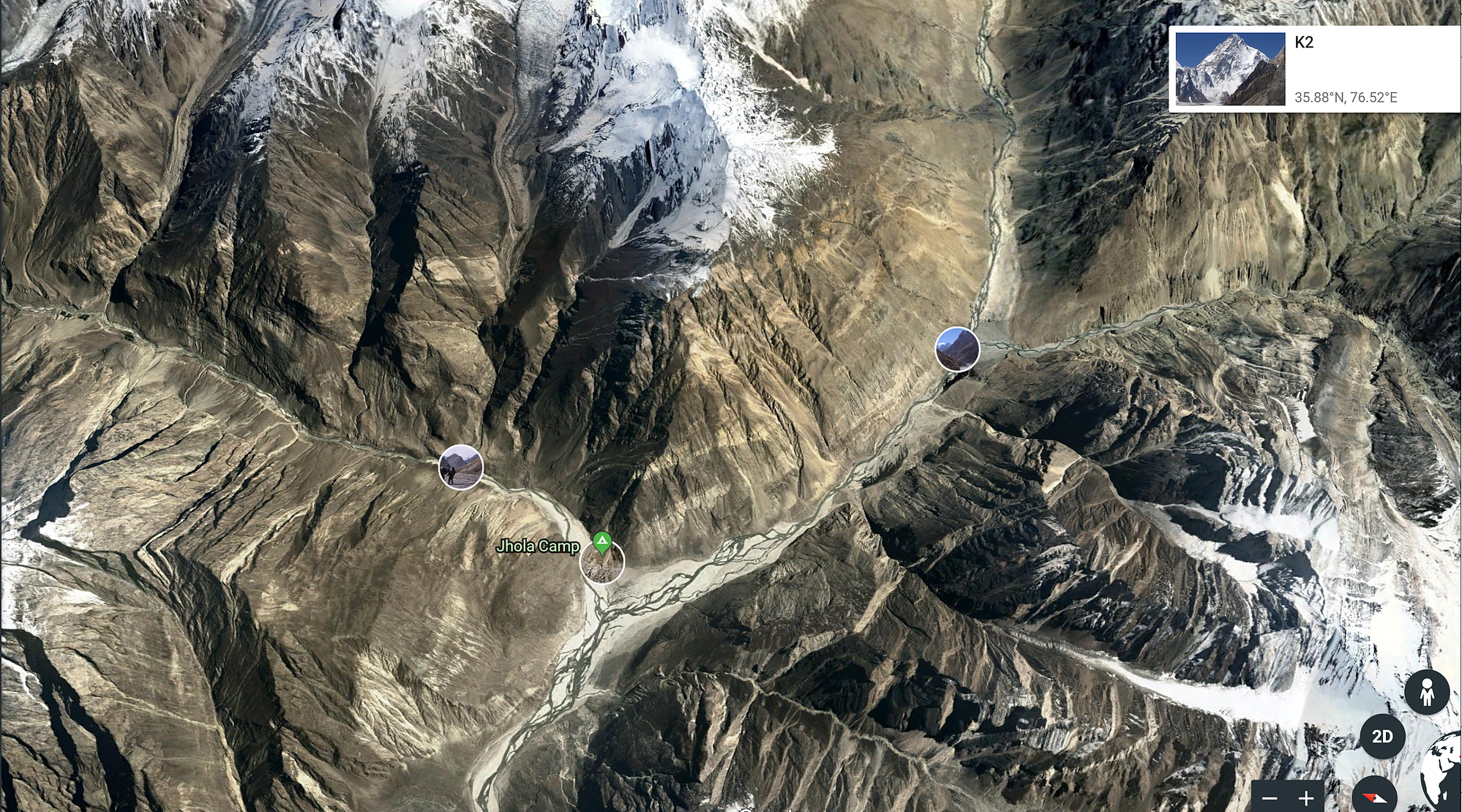
There is not alkaline rocks around Baltoro Glacier where K2 rock is found.
Trango Towers seen from Baltoro Glacier.
Terrain around Baltoro Glacier is conformed by materials from the Baltoro Plutonic Unit (granodiorites and monzo and leucogranites) with minor zones of gneiss, all of them are acid rocks non-compatible with sodalite genesis.:
These granite mountains are known as The Cathedral, seen from Concordia, the center of Baltoro Glacier:
Here for sure the blue is not sodalite (nor azurite!)

21st Jan 2014 01:52 UTCMichael Hatskel
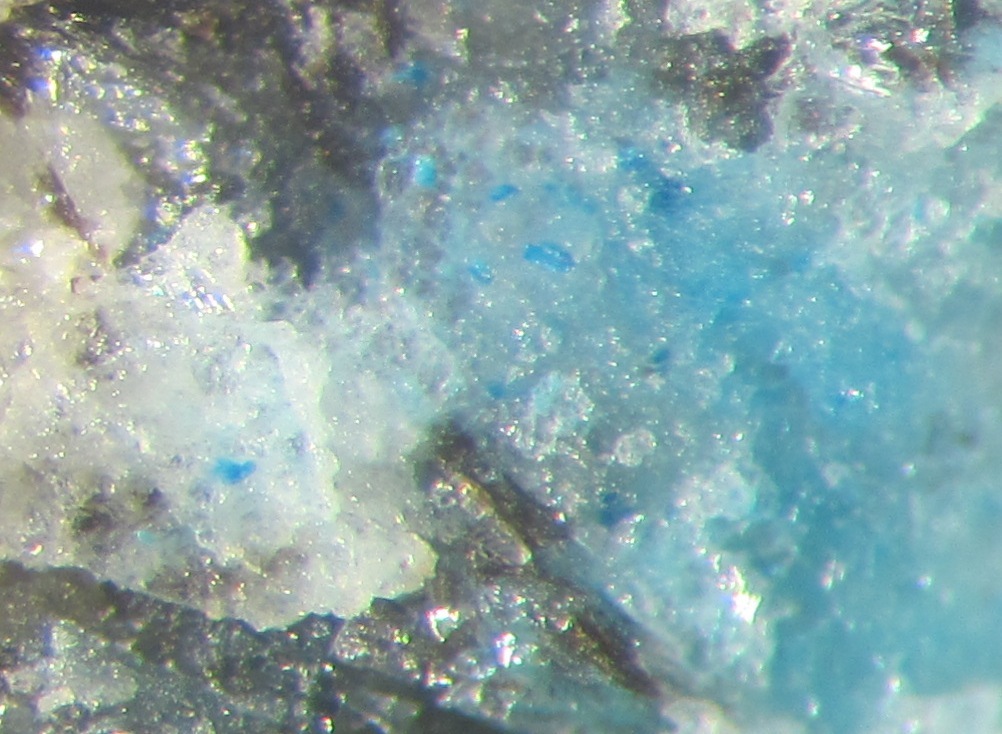
I am surprised that there were no microphotographs of the "blue stuff" posted in this thread, so I am posting my own quick and dirty pics taken through the scope ocular. One pic shows separate inclusions, the other shows something like microcrystalline grains of both blue and green color.
I was able to expose some larger inclusions and extract then from the feldspar. When brought in contact with HCl on a white plastic surface, the blue component disappears (gets dissolved) within 1-3 seconds, depending on the particle size. As Alfredo correctly stated (when was he wrong? :-)), the amount of the mineral was not enough to produce visible gas bubbles - again, visible at 70-100x magnification. However, the mineral particles were moving very fast and chaotically inside the acid drop, which typically points to the gas evolution propelling the particles.
In summary, my observations seem to support the "K2 Blue" identification as azurite.
I was actually hoping that it was some copper silicate, based on its residence inside the feldspar.
Some additional comments:
1. The "spots" are actually 3-dimensional aggregates of the colored feldspar grains, not flat 2-dimensional spots.
2. No residual metallic grains were observed in my specimen.
3. No distribution pattern was observed for the spots distribution in hand specimens. I have inspected a full crate of very large K2 rocks, and could not devise any system pattern.
4. None of the bright blue inclusions had any discernible shape: they are basically anhedral grains or flakes or shapeless aggregates.
21st Jan 2014 17:25 UTCReiner Mielke Expert

21st Jan 2014 19:12 UTCMichael Hatskel
Those green patches are very rare in my specimen - just in 2 or 3 of the blue spots. All of the coloration is absolutely predominantly blue. I included the second photo just because of the green presence there, but it is not characteristic or widespread at all.

21st Jan 2014 21:50 UTCHenry Barwood

21st Jan 2014 22:25 UTCAlfredo Petrov Manager
Metallic (uncombined) copper is soluble in feldspar-rich silicate melts and exsolves during crystallization of the feldspar (as ones sees in "Oregon sunstone" gems, for example).

21st Jan 2014 22:32 UTCMichael Hatskel
One of the possibilities is that the enclosing feldspar is a recrystallized product of the metamorphism which also brought in the CO2 to create a carbonate.

21st Jan 2014 22:57 UTCHenry Barwood
I can't believe this thread is still stumbling along!

22nd Jan 2014 00:09 UTCAlfredo Petrov Manager
22nd Jan 2014 00:54 UTCReiner Mielke Expert

22nd Jan 2014 01:15 UTCHenry Barwood
Recently Julian Gray of the Tellus Museum in Georgia has discovered interesting carbonates (calcite and REE carbonates) associated with the granites that host the amethyst localities in central Georgia. I have also found significant calcite present in zoisite containing granodiorites from Alabama. Carbonates are likely more common than recognized in granites and leuocogranites.

22nd Jan 2014 05:43 UTCFranz Bernhard Expert
Franz Bernhard

12th Mar 2014 18:56 UTCshah
Ps: these are some pics i have got.It is also known as Raindrop Azurite and jasper k2 azurite(jasper not make sense but its more famous name) and it has some Malachite spots as well in it which i have seen in some of the pieces personally.
12th Mar 2014 20:34 UTCSteve Hardinger 🌟 Expert
I stated that gas was released upon immersion in acid, but I did not state the nature of the gas. Perhaps it was CO2 or maybe just entrained air.
Until the chemical composition of the gas is verified, we cannot use its composition as evidence for or against any carbonates.
In addition, I did not say that gas was released from many samples; I tested only one. Perhaps the gas is entrained air, and is not present in all samples.

23rd May 2014 19:19 UTCKo-Chun Huang
To see a long discussion here, I cannot eliminate curiosity on this interesting rock,
so I obtain a small sample which I did some SEM work to see if we can get some further info on this rock,
and Hope this help to resolves the nature of the K2 Blue.
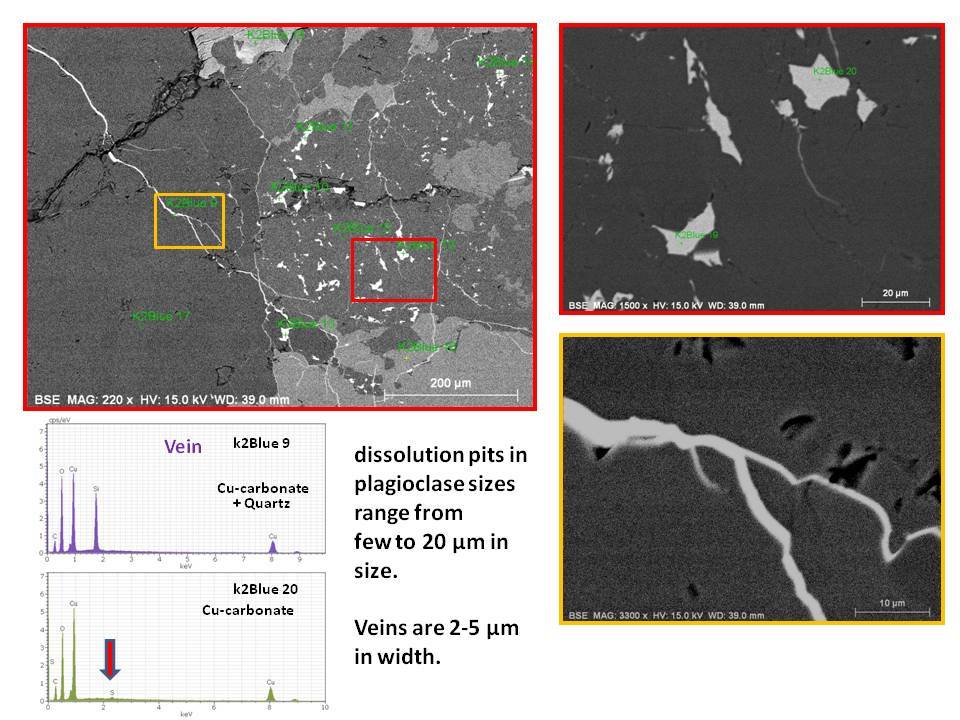
I found several features of K2 Blue sample:
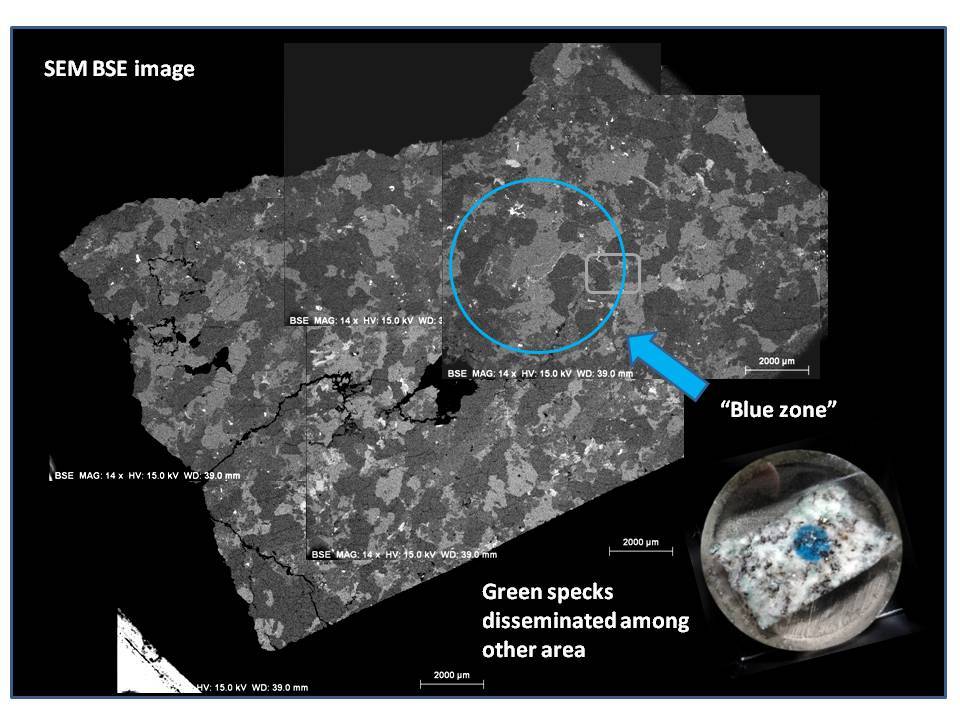
(1) The blue mineral are found as copper carbonate (azurite) as indicated by EDS data.
(2) Azurite occur as micron sized veins in and around quartz or K-feldspar grains (Size range around 2-5um in width) or as small pockets (5-20um) in plagioclase which I suspect them as dissolution voids formed during hydrothermal alteration.
(3) Plagioclase which hosts void filling copper carbonates has composition close towards albite, which supports Dr. Barwood's observation.
(4) Very minor amount of Sulfur was detected in these secondary copper mineral, makes me wonder the possibility of pre-existing copper sulfides in the rock, which got alterated by late stage CO2 rich fluid event.
Overall, I think it explains why these blue spots seem overprinting the original rock textures of the granitoid, because they formed later than the rock matrix minerals. This can be evident by occurrence as veins and void filling.
Due to very small nature of these blue minerals, it will be difficult to see bubbling reactions with HCl, and it will be hard to tell base on hardness or other physical test.
Cheers
Ko-Chun Huang
Lab of Micro-Nano Mineral Science
Department of Earth Sciences
National Cheng Kung University
Tainan, Taiwan.
23rd May 2014 19:31 UTCRob Woodside 🌟 Manager
24th May 2014 01:20 UTCReiner Mielke Expert

25th May 2014 15:30 UTCKo-Chun Huang
even intended to do EBSD structure determination (which would need more time).
But I think so far to all the experts here answered, my result is pointing to the same direction, and I am happy with that.
Thanks Rob, I am happy that I can help.
Thanks for the suggestion Reiner Mielke, I will prepare a better one to put on mindat soon.
BTW, does anyone actually know where it come from??
Ko-Chun Huang
Lab of Micro-Nano Mineral Science
Department of Earth Sciences
National Cheng Kung University
Tainan, Taiwan.

25th Sep 2014 03:30 UTCbre

22nd Apr 2016 05:35 UTCTJ Taylor

22nd Apr 2016 06:19 UTCAlfredo Petrov Manager
22nd Apr 2016 15:11 UTCSteve Hardinger 🌟 Expert
22nd Apr 2016 16:22 UTCRob Woodside 🌟 Manager

23rd Apr 2016 17:28 UTCFlorian Baur
Regarding to the origin, there's a post by someone saying he's a close relative to the main (only?) supplier of the material:
http://www.mindat.org/forum.php?read,6,304299,318734#msg-318734
For some reason there was no reaction to that post. Maybe the author can be contacted?

2nd Jun 2016 15:07 UTCAmy

2nd Jun 2016 15:42 UTCAlfredo Petrov Manager
Anything used to remove the oils must not affect azurite. You could try an organic solvent like gasoline, but it might take a very very long soak. This is a case where an ounce of prevention is definitely worth a pound of cure. Best just make sure a sealant is applied to any future pieces you acquire, Amy.
2nd Jun 2016 19:25 UTCRob Woodside 🌟 Manager

4th Jun 2016 17:01 UTCAmy

5th Jun 2016 16:57 UTCD. Peck
I have used diluted epoxy to seal rock chips from which I have cut thin sections for study. In those cases, I soaked the chip for 24 hours, removed it from the solution and heated it for another 24 hours.
I would think that epoxy diluted with toluene (what I used, 5 parts toluene to 1 part epoxy), would work for you. The diluted epoxy soaks into minute voids and cracks that open to the outside. The heating sets the epoxy and drives off the toluene. It does not have to be real hot, just a good warm place will do. I have never used acetone to dilute the epoxy, but I think it would work and it is easier to get. If you try this, be careful with the acetone or toluene, it is highly flamable.

6th Jun 2016 08:50 UTCAmy

6th Jun 2016 18:36 UTCDana Morong
Amy, I was going to send a PM (Private Message) re ASD but you don't have PM (may not be registered on mindat.org?).
Anyhow, enjoy your minerals. They can be therapeutic, but I'd be careful of the volatile chemicals sometimes used in cleaning or treating them.

14th Dec 2016 15:39 UTCAnonymous User
Anybody willing to get this stone may contact us at: thestonerspk@yahoo.com
The FOB Karachi (Pakistan) rate is US$60.00 per Kg in rough shape boulders upto 30Kg in weight. MOQ is 20Kg.
Regards,
Ahmed Anwar Amin
Cell: +92-313-2753209 (WhatsApp / Viber)

14th Dec 2016 15:44 UTCAnonymous User
The interesting articles on this stone are:
http://geology.com/gemstones/k2/
https://www.linkedin.com/pulse/k2-jasper-unconventional-gemstone-kathleen-marino-g-g-
Hope these will be read-worthy for those who are interested in this stone.
Regards
Ahmed
16th Dec 2016 05:36 UTCGregg Little 🌟
26th Dec 2016 02:10 UTCRalph S Bottrill 🌟 Manager

10th Mar 2017 00:11 UTCAnonymous User
10th Mar 2017 03:51 UTCSteve Hardinger 🌟 Expert
10th Mar 2017 05:43 UTCGregg Little 🌟
We'll see what others chime in with but in the mean time try some dilute HCl on the blue mineral to see if it effervesces, under magnification if necessary.

9th Apr 2017 20:19 UTCShah Abbas
Regards,
Shah

28th Apr 2018 17:54 UTCsteve m johnson
Close look showed the stain was not always controlled by mineral edges or aggregates. Clearly the stain crosscut borders. In posted pictures posted I could see signs of two distinct mineral staining events that seemed exclusive...one, the earlier was cloudy green and looked like several coloration fronts had merged into a larger one with stronger colors clearly present along an irregular and diffuse pseudo bulbous "frontier" with a crude center that appeared depleted of coloration. Apparently superposed on it was the darker blue forming the distinct separate splotchy features. Most of the blue could be associated with tiny dark deep blue aggregations at 5x magnification. The specks seemed irregular and no signs of a radiating or acicular structure were obvious. Blue speck centers did not host any particular mineral and the distribution of small dark granules seemed irregular within the blue color field. Anyone with a dropper bottle of sulfuric acid and a nail could demonstrate the presence of copper with a few drops on the blue field and scratching the wetted spot with a nail or even the tip of a rock hammer. The presence of copper would be indicated immediately by copper plating on the steel. That test seems to have been ignored in favor of many exotic tests some of which showed copper percentages exceeding 1%. Such concentrations of Copper are considered Very high....as mining in Chile, where I work proceeds at levels averaging 0.65% Cu down to as low as about 0.35% Cu. There were no signs of sulfide mineralization in any of the polished specimens I observed, clearly suggesting that we were well within the hematite mineral field but the lack of hematite staining and the presence of quartz, biotite and feldspars were surprising, suggesting exposure was so rapid that weathering had not proceeded significantly. The granites are hard and show no signs of clay or even significant argillic alteration. The granophytric textures exhibit a noticeable foliation in many specimens but with few obvious signs of fractures, veins or other typical signs of resurgent shallow intrusion such as healed straight brittle failure veins or even bent veins that were plasticized through re-heating. Given the presence of the tartan plaid twinning and some of the rock analyses I am inclined to make the case that the granitic material with the blue splotches reflects rapid exposure of the potassic core of a differentiated igneous intrusion that has been so oxidized that sulfides have been entirely transformed to oxide zone minerals. The oxide zone minerals are most likely found as azurite/malachite probably after chalcocite and maybe after primary bornite (!) in my opinion (given the way chalcocite shows up in my area in Chile...tiny spots of roundish sectile material after Covellite after Chalcopyrite).
The apparent lack of sulfides and the lack of any sort of yellow or brown coloration associated with iron staining from alteration of chalcopyrite and other iron phases. The carbonate bearing granitic assemblage appears well within chlorite/propylitic zones and is probably associated with a carbonate rich phase of core potassic alteration.
Carbonate phases are entirely stable within the core of a cu porphyry system depending on the particulars of the geochemical system. In this case, assuming the material represents a magmatic system, which I strongly suspect, the parameters are oxide rich and..apparently...very low sulfidization. The association with porphyry core mineral aggregations, biotite and k-spar without clays or obvious silica flooding is supportive of the chance that intrusive projections...dikes/sills and plugs etc formed where silica saturation was well balanced. The mineral mix may have still been within the solution solubility of the melt. (Veins would be expectable only during strong silica oversaturation.) Because of the lack of veins, the material separated into disseminated metal phases that were not transported much and the material was emplaced in the plastic zone...below the zone of brittle failure.
The apparently simple lithology presented in the specimens posted suggests the K2 Jasper material may be a one event "shoot" coming out of a larger body that has not been recognized yet.. No signs of incorporation of fragments from the larger body within the Shoot are reported or visible in photos but several 1+ cm xtls of k-spar were visible in hand samples suggesting something is present within the magmatic system that we know nothing about but which deserves consideration. The even distribution of small colored grains, green and blue (azurite and malachite probably) ignoring the distribution and extent stained areas suggests the colored grains may very well reflect the remains of so called primary igneous disseminations (probably sulfides) formed during solidification. Overall, the samples suggest the geological exposure of parts of a mineralized system. The presence of muscovite and biotite are consistent with potassic associations and the presence of microcline/k-spars certainly appear diagnostic.
I would attribute the green and then the blue staining to "diffusion" within a "hard" water system at depth. Then, rapid uplift during mountain would change things. My guess is that the system went dry several times during uplift. The dry conditions arrested diffusive ion migration and froze the spheroidal geometry of the leading edge of the stains. The presence of weak hexagonal or pentagonal stain outlines is present but inspection shows the outlines are not obviously associated with mineral edges. I speculate that the outlines may be the result of "mechanical" influences. I propose that the "edged" geometric features visible on some samples reflect geomechanical ion transport. The system I suggest to explain some oddities is the result of some sort of mechanical stress/strain activity, likely during heating and cooling events during crystallization of particular phases underground. Crystallization releases heat right? Anyway, the process I propose in porphyry granite systems undergoing solidification are a shrink/swell pattern that is similar to freeze/thaw cycles that are responsible for "patterned ground" at the surface in relict tundra/cryokarst areas. My bet is that the unusual diffusion occurs in well preserved "porphyry granite" core material with somewhat unusual lithogeochemistry while the hot melt rock is "breathing" a bit.
The distinct dark to light pseudo spherical blue stains reflect the latest ion migration event and from what it appears...that phase was arrested before it dispersed as much as the green tinted cloud-front migration event that is sometimes visible.
I could go on a bit but I want to go buy a few samples to show to my friends and see if they can come up with a better story.
28th Apr 2018 22:11 UTCGregg Little 🌟
I find genesis endlessly fascinating. The apparent oxygen activity in an igneous melt is really unusual especially when we usually think of copper carbonates in the oxide zone of mineral deposits. I am very curious about what your friends will come up with.
28th Apr 2018 23:59 UTCRalph S Bottrill 🌟 Manager

29th Apr 2018 02:23 UTCsteve m johnson
The alteration associated with the K2 Jasper is right in front of your eyes. Thin sections are not very helpful in my opinion but field relatios are. Take some sodium cobaltinitrate and stain the material. Chances are you will not only see the big k-spars get altered but the interstitial material. The ICP work showing a few percent K is strange given the observation of microcline in some thin sections. Anyway, microcline is the key....you don't need much to signal core material is present just a consistent paragenesis showing the right minerals co-exist. It is entirely possible to miss extreme potassic alteration on casual inspection. Biotites and feldspars can look fresh as can be and boom...the rock runs 14% potassium. The potassic zone is typified by biotite and k-spars not argyllization of minerals. Interestingly, the discovery of primary bornite in core is now considered to indicate important things... Whole exploration programs have been started in chile just because of the discovery of primary bornite in "condemnation cores". The discovery is called Escondita North I believe. It was recently reported in a small abstract. For what it's worth, the stability field of carbonates extends to great depth...essentially to mantle or lower mantle depths. Those are the depths of carbonatite genesis...very deep. Phosphates are stable at very deep levels. I am plagued by the presence of microscopic covellite that nobody noticed. I went over core using 30x and 40x and saw stuff that nobody even noticed even though they found big chunks on the surface. After looking things over in plain light at 40x or so Then maybe thin section...but really, in 25 years of mineral exploration, I have never seen much use of thin sections for anything but mineral processing. For example, where I am, conditions are so dry we are seeing blue chalchanthite seeping out of the rocks, probably from a perched exotic copper/supergene deposit sitting at 16,000 ft or so where access is limited. (Strangely, there is as strong a topographic anomaly were I am in Chile as at K2. My property runs to 18,000 ft...and is...you guessed it...right on the watershed crest between Argentina and Chile as are many other mines. Of course, K2 is right on the divide between China and Pakistan. Strange...these hydrological anomalies. There ought to be porphyry systems in the Himalayas.
Regarding tourmalinization....the company Teck has some nice samples from core at one of their mines showing extensive tourmaline is fractures. I have never seen any work relating the existence of tourmaline with actual copper mineral segregation but Borax is good at separating gold from its matrix...maybe tourmaline plays a similar role.

29th Apr 2018 02:32 UTCsteve m johnson
Basically sulfide rich melts have a solubility for copper that is...what 100x...1000x? Obviously copper carbonates might exist but I do not think the hydrated ones are stable at depth. If a system doesn't have enough sulfur to hold copper the system is expected to be barren. Mafic rock contains much more sulfur than silicic rock by about 100x. The process of separation of copper requires a magma that can soak up and expel its load of metals. I do not think there is much work on carbonate systems so I just have to guess that the disseminations in the K2 Jasper are replacement phases from sulfides. Where I come from you want to see hornblende being destroyed to make a fertile system. The hornblende provides the water and maybe some of the metals. The transformation from hornblende to biotite is one of the indicators of vectoring toward the potassic core. I did notice a few unidentified black minerals within the k2 jasper pieces. I did not take the time to see if they had distinguishable features but if they were hornblendes, I would not be surprised. If they were pyroxenes...I would be very surprised.
29th Apr 2018 04:16 UTCMatt Neuzil Expert
29th Apr 2018 11:22 UTCReiner Mielke Expert

29th Apr 2018 13:46 UTCAlfredo Petrov Manager
And, in addition to the one pointed out by Reiner, here are a couple more localities for primary native copper included in magmatic feldspars:
https://www.mindat.org/photo-587489.html (as Matt already noted)
https://www.mineralienatlas.de/lexikon/index.php/MediaDataShow?backlink=1&lokationid=12099&galerie=mineral&nobildertyp=22
https://www.mindat.org/photo-846170.html
It seems that copper exsolves from feldspar on cooling.
29th Apr 2018 14:42 UTCGregg Little 🌟
One of Steve's very interesting observations is the altitude at which this azurite staining occurs both in his area and at the K2 azurite site. The relative humidity of very cold air can be high. Further, as Steve indicated, field work needs to be done to figure out the deposit's spacial relations. One unanswered question about the K2 copper carbonate staining is whether it is superficial or pervasive with depth. Supergene enrichment at high altitudes could have a whole different character from those typically encountered at lower altitudes.

30th Apr 2018 20:51 UTCsteve m johnson
Thin sections would be OK but I bet you could find black specks of tourmaline in that rock at 40x. Polished faces should eventually reveal the existence of some sulfide...but if that sulfide is covellite and blue...you might not even see it. Careful examination might show the paragenesis of azurite after covellite after ....bornite. Usually covellite suggests high sulfidization but in fact, covellite can form surficially. It has been identified as a kind of "rust" on some tools.
For what its worth, eroded out supergene material does not go away. The copper does not just disappear, it can and has be captured naturally and accumulated as "exotic copper accumulations". I think there are several places where that has taken place where I work, except it is Digenite way up high, flushed out down where the drill roads are as chalcanthite. Wish my project area were lower and not half a world away but then someone else would have grabbed it before me. Anywhere there are several examples of folks failing to recognize the potential of "exotic" copper concentrations in the literature but one interesting one has to do with Richard Sillitoe's recommendation to explore a flat area adjacent to an active mine, probably in Chile. On his say there was a lot of supergene copper discovered at shallow depths in the flats using blast hole drills. Apparently geologists and mine personnel had driven past the deposit every day for some 60 years without even thinking about the possibility.
Attached is an example of the covellite after chalcopyrite from an online image, the other image shows my area with what I have evidence of... a perched supergene deposit.leaking abundant blue copper mineralization from space shots. On the ground .we identified lots of blue stuff in the field as calcanthite probably after chalcocite or possibly supergene digenite. Some samples in the field ran 69% Cu!.
30th Apr 2018 23:14 UTCGregg Little 🌟
Is that the drill pad and road on the left to upper left of the photo? I can't see the rock hammer for scale. Treeless slopes really are the explorationist's friend.

1st May 2018 01:30 UTCsteve m johnson
Like in "farewell to tarwathy" by Judy Collins....like the cold coast of greenland....in Chile
the winds blow through passes that are three miles high...
but the air is so thin it is as worthless as breathing the sea
indeed,,
there are no song birds in Chile to sing to the whale....and there is no habitation for a man to live there
but we all hope to find riches a hunting the whale.

1st May 2018 07:27 UTCsteve m johnson

1st May 2018 08:07 UTCAlfredo Petrov Manager
1st May 2018 11:57 UTCJosé Zendrera 🌟 Manager

3rd May 2018 07:28 UTCsteve m johnson

6th Nov 2018 00:52 UTCTim Jokela Jr
6th Nov 2018 02:03 UTCFrank K. Mazdab 🌟 Manager
I analyzed it... it's my thin section FKM-105 (https://www.rockptx.com/fkm-101-to-fkm-125/#FKM-105).
The blue sunbursts do contain tiny scattered azurite, but not very much. If one looks closely, it's possible to see tiny darker blue specks within the larger blue sunbursts. Those tiny blue specks are the azurite.
As for the rest of the blue material, most of their volume is a fine-grained admixture of albite+quartz with a combined copper content of ~1200 ppm Cu. Because the albite+quartz mixture is intergrown on a such a fine scale (1-5 microns, comparable to that of the microprobe beam diameter), it was difficult to get an albite-only (or quartz-only) analysis, although from the BSE imaging the mixture in my sample appeared to average about 70% albite & 30% quartz. So it's unclear if the 1200 ppm Cu represents solid solution in the one of the minerals, or alternatively the presence of some sub-micron discrete Cu mineral (more azurite?; chrysocolla? something else?) just not visible in the BSE imaging. In any case, that small amount of Cu, whatever its nature, along with the scattered tiny azurites, are enough to color the sunbursts.
What's also interesting is that many of the minerals present contain measurable Cu: muscovite & biotite (~450 ppm Cu), chlorite (~1000 ppm Cu), titanite (~650 ppm Cu) and epidote (Cu not yet measured). There was no evidence of a pre-existing Cu sulfide to source the azurite and other Cu enrichments, although it's possible it was there and was just obliterated during low grade metamorphism or propylitic alteration and then redistributed into the minerals present now.
Frank
6th Nov 2018 02:18 UTCKevin Conroy Manager
Attachments
You need to be logged in to view attachments.
6th Nov 2018 19:16 UTCTim Jokela Jr
5th Nov 2019 00:41 UTCJosé Zendrera 🌟 Manager
"K2 granite" can not come from surroundings of K2 mountain simply due to transporting cost. K2 base camp is only accessible after a day long hellish 4x4 track followed by a hard ten days walk. Even with a lower price that is paid by alpine expeditions for equipment porting, this rock could not be offered in Skardu or Karachi by tons for a few dollars/kilo.
In other hand, I've seen many minerals for sale in K2 approach route but never this blue dotted granitoid.
As stated by Shah Abbas up in this thread, the stuff comes from somewhere near Khaplu (or Khapalu), 75 km south from K2 mountain, necessarily in a place not far from a truck accesible piste when is so widely distributed, even in big pieces.
I think K2 is in this case just a commercial name taking advantage from a famous peak and promoting a mystery about his origin that after all has been quite succeful, as proves this long thread itself.
By the way, Khaplu is closer than Skardu from K2 but they profite very little from mountaneering tourism because people in route to K2 and other eighthousanders in the area mostly go from Skardu up the river until Baltoro glacier and only a few on the way back dare to cross Gondogoro Pass returning to Skardu via Khaplu.

11th Oct 2023 16:19 UTCAfzal Gulzar
yes, you are right. K-2 granite is the commerial name. The mine is not located in the k2 area. The mine location is in Ghanche district. As i already visited this year.
12th Jul 2021 10:59 UTCJeff J Jessie
Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 18, 2024 22:53:28
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 18, 2024 22:53:28