Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Identity HelpUnknown green stone?

15th Sep 2017 10:14 UTCRio Nevin
The seller said that this was idocrase (vesuvianite, or maybe californite since it's massive?) but the local lapidarist who cut this thought that it was serpentine.
These rocks have different shades of green: rather vibrant green near the black inclusions (largest piece), olive green (2nd-largest piece), pale green with very slight hue of blue to white (all pieces).
The SG is very close to if not exactly 3, which is heavier than serpentine but lighter than californite. Maybe neither?
Hardness is between Fluorite and Labradorite (closest ones that I have).
Also, it cleaves very easily, and it cleaved when cut by the reckless local lapidarist. The cleaved side looks a bit silky (while the rest is waxy and translucent) and can be "peeled" with my fingernails with some difficulty (third image shows the peeled fragment).
Any help in identifying this would be appreciated, many thanks!

15th Sep 2017 14:18 UTCKeith A. Peregrine

15th Sep 2017 15:15 UTCRio Nevin
-------------------------------------------------------
> Rio, do you know the origin?
I'm not sure, most likely Indonesia. I've heard of people talking about both jade and serpentine from Aceh region, and idocrase/californite from Sungai Dareh / West Sumatra. The seller himself sold this as idocrase so it *may* be from West Sumatra.
16th Sep 2017 10:53 UTCJoel Dyer
The material would seem to be too soft for vesuvianite, which I don't think it looks like that much. Also, if you measured the hardness correectly, it should be too hard for serpentine minerals. Does an iron nail or steel knife scratch the material really easily?
Thinking of jade, especially nephrite, it is often quite splintery but tough, and (semi)fibrous. Your material would seem to be more splintery and of this type, than some sharply, straight-cleaving stuff.
An experienced gemmologist or mineralogist should be able to tell you what it is, or if you think it worthwhile, it would be very easy to analyse a small chip of it with many different methods.
cheers,
Joel
16th Sep 2017 17:25 UTCOwen Lewis
Just looking at your pics, nephrite jade (esp. from NZ) pops into mind - which is no way to be certain of anything ;-)
Taking it further, your SG is good for nephrite but much depends on the accuracy of your weighings. For what ever unit of mass you are working in, you weighings should be accurate to (at least) three decimal places - which should assure you accuracy in SG calculation to the two decimal places desirable for differentiating minerals.
Nephrite hardness is 6.0 - 6.5, the same as for feldspar.
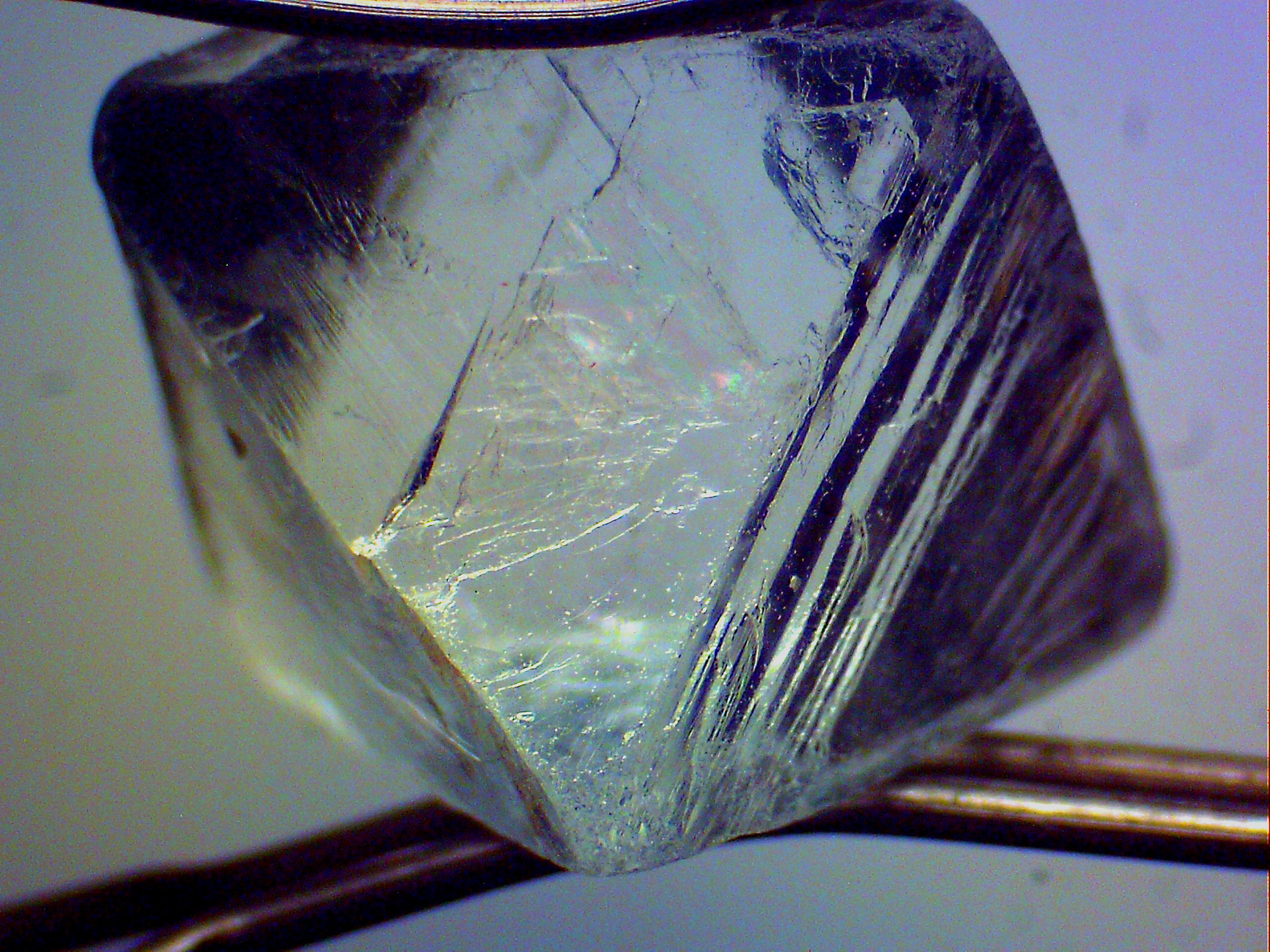
However, NEPHRITE NEVER EXHIBITS CLEAVAGE! Absolute rule. You *sure* you have cleavage and not a fracture? Can you post a good sharp photo of the cleaved surface that shows the quality of the cleavage? The small chip you show does not look cleaved to me - but one needs to see a surface that's illuminated at a grazing angle, to emphasise imperfections in the (cleaved?) surface. To show you what I mean, here's two pics that show (in fluorite) a natural 'perfect' octahedral cleavage and (in hydroxylapatite) indistinct basal cleavage. Note that, in the apatite pic, there is a second incipient basal cleavage. Though both only incipient and imperfect, that cleavage is still strong and complete enough to reflect back (upwards from that incipient cleavage) the 'grazing angle' light coming from the right and above the cleavage line, making the crystal above that line well-illuminated as though from within (which it is in this set-up) with the light thrown back upwards from below. Beneath that line the crystal is much darker (but still visible) because of the relatively small amount of light passing the incipient cleavage. Pictures can explain beyond words sometimes.
Nephrite's prime physical characteristic is its extreme toughness (resistance to cracking apart from a hammer blow). As Joel says, when it does break open, the fracture is splintery and may show a pattern of matted actinolite and tremolite fibres (that give it its toughness. Here's a pic of a small rough-sawn nephrite slab tripped a little in the sawing, probably because of imperfection in composition.
Since you seem to have had some cut into a polished cube, if you would like to send me a flat-polished piece, plus the small rough flake, I'll run a series of tests for you, including an accurate re-do of the SG and measuring the refractive inidices which should put the matter beyond doubt. No charge, just return postage. PM me an e-mail address for you if this seems useful to you. ,

16th Sep 2017 18:08 UTCRio Nevin
I'm not sure if I measured the hardness correctly since seeing the scratches is quite hard on this stone. (And obviously, my lack of my experince as an amateur)
I haven't thought if it is a cleavage or fracture (splinter), but whatever it is, it's not straight cleavage thingy but rather wavy, perhaps it is a fracture (or even some kind of "grain boundary"?)
One interesting feature is that one thin fragment had a huuuge crack halfway along the length (after cut by the lapidarist), and it is quite wide so that I could put a sheet of paper in it. Oddly it was hard to use my fingernails to break it apart along the half-broken fragment.
I will post more detailed photos tomorrow morning (including the crack and "cleavage"), it's almost midnight here...
Oh and since I'm also unfamiliar to technical photography, does "grazing angle" mean that the light comes to the direction of the camera, but almost parallel to the observed side? (Perpendicular to camera?)
The SG measurement were actually done twice, once on the largest piece and then once on the next-largest one, both using a digital scale accurate to 0.01 gram. The first yielded 33.81g/11.24g=~3.008 while the second yielded 20.22g/6.74g=3 (exact 3). To me this indicated a pretty legit measurement :D
Thanks for the test offer Owen, I appreciate it very much.
Cheers,
Rio.
16th Sep 2017 19:35 UTCOwen Lewis
'Grazing angle'. Angle between the surface being photographed and the beam of light illuminating the subject is about 5 - 10 degrees only. A camera lens to subject surface of close to 90 deg is assumed. Play with small movements of the light source to get the best effect (i.e. 'mountains' casting shadows). The light source should also be collimated so that as much of the light as possible is thrown forward in a more or less parallel beam. A cheap solution to this is something like a pen-light torch that reflects most of the light from a parabolic mirror behind the lamp and that allows the user to adjust the spread of the light beam cast forward. The best solution is light delivery through a fibreoptic with a small lens over the exit point that focuses all the light into a parallel beam.
SG. Sound good to me. The SG reference data I use for nephrite is 2.95 (+0.15/-0.05) The SG of my reference specimen is 3.03. As nephrite is a solid state solution of actinolite and tremolite, an acceptable value range for SG is the best one can ever do (with the observation that 2.95 is the most commonly reported 'spot' result).

17th Sep 2017 05:49 UTCRio Nevin
close up of specimen under diffuse sunlight,
and,
close up specimen under LED light (is this grazing angle?).
The photos are taken with an android phone with the help of a loupe.

17th Sep 2017 06:13 UTCRio Nevin
Despite of the crack, it's still tough and did not break apart easily.
Here's the detail of the surface:
The piece in image 2 had fallen from my desk, resulting in a white "bruise" visible on the bottom right corner of the piece in image 2.
And here's the detail of the bruise:

17th Sep 2017 06:20 UTCRio Nevin
^does not seem to help much?
Anyways, maybe all of these additional photos can help?
17th Sep 2017 06:32 UTCJoel Dyer
You've done good work! All those extra tests and photos are excellent, and everything seems to point towards your material being nephrite, especially since Owen as an experienced gemmologist seems to consider this as well.
Being an "analyst" myself, I can't confirm 100% from pictures it is nephrite (=actinolite-tremolite with inclusions of other minerals) without analysis, but I'd be rather surprised if it isn't.
Cheers,
Joel
17th Sep 2017 18:35 UTCOwen Lewis
greenman_graze_1.jpg Yes, that's lighting at a grazing angle. And that is no cleavage you show there - so we are still OK with a nephrite ID- everything even down to the bruise appearance.
The microscope shot is good as well, but there's an anomaly. If I click on the image to enlarge it; the image becomes seemingly peppered with fine green, blue and some yellow spots that are *not* digital pixellation but could be dye transfer grain, if you made the image on colour film stock before converting it to digital format for web display. Any ideas? My guess is that it is a photographic artefact and n characteristic of the stone but....? Also (and not that it will help with the ID), do you know what the field of view (FoV) is?
I think I'd be happy with making a nephrite call but, for certainty, you will need either an XRD/XRF printout or confirmation of your SG determination with a freshly calibrated scale and (if you have a piece with a polished plane surface) determination of the 2 RIs, the birefringence and optical character. of the crystal.
A footnote on SG determination. Getting an accurate dry weight relies only on the accuracy of the scale and its proper use (i.e. taring before weighing). Obtaining really accurate wet (under water) weight requires, in addition, a different weighing method to the one you use and slightly more complicated arithmetic to calculate the SG. Basically, if as a mineralogist, you want to determine (as Archimedes once did) SG's of kilo sized chunks of a mineral, depending on the purity of your specimens, Archimedes's method is usually good enough. However, if your specimens are less that around a gram in weight (and there is good reason to choose such small-sized specimens to work with), then a more accurate scale and a different method of wet weighing is required, the latter resulting in slightly more elaborate arithmetic (who cares! push a key and a suitable formula embedded in a spreadsheet cell or database record, will spit out a 100% correct solution in less than a millisecond, every time).
For an absolute check on whether your current SG determinations are good, Get a small, clean specimen of rockcrystal/amethyst/citrine. That material will invariably return a good SG determination that is 2.65 +/0.01. If you get any other value then there is something awry with either the accuracy of your scale or your technique in determining the weight of the water it displaces. Get a bit of quartz (not chalcedony or smoky) and see what accuracy you get. Should you happen to have one to hand, a clean diamond (SG 3.52 +/- 0.01) is another absolutely reliable test piece. Without such certain checks before every session, the tester is pretty much in the land of wish-fulfillment (belief that neatly printed numbers never lie!).
Really reliable SG determinations are (IMHO) one much underused tool for specimen identification available to the amateur mineralogist/gemmologist.

18th Sep 2017 03:42 UTCRio Nevin
For the microscope shot, the FoV is around 1.5 mm (I put a ruler under the microscope lol). Also, I put my phone directly above the ocular lens of the microscope, where an observer's eyes should be. I've used this method to easily take microscope photographs. So if what you meant was an image artefact, it should be a digital artefact. Perhaps it's the "graininess" of a high ISO-photograph, since the light was not that bright when doing the shot.
For the SG, the method I use seemed to be unreliable for small samples (even though I improvised the method by using a rig to hold the sample still). 10 gram amethyst yielded SG of 2.64 and 4 gram quartz yielded 2.70, 2.65 and 2.69 (on the same piece, done 3 times). But for larger samples (more than 10 grams) it seemed to be more reliable.. e.g. 20 grams of chalcedony yielded 2.62 SG.
I am planning to send you some samples Owen, I'd be glad if you'd do the tests. I have the polished piece ready to ship, now just need to ask the local post office about the shipping fees... I hope it's not too expensive, otherwise I would consider sending it to a local gem lab instead :(

18th Sep 2017 04:05 UTCDoug Daniels

18th Sep 2017 07:08 UTCRio Nevin
Or maybe I should ask other delivery services.
18th Sep 2017 09:32 UTCJoel Dyer
Are you looking at getting every single gram of the material "certified" by a gemmologist or other lab? If not, why not just send a small chip of each piece somewhere to a gemmologist, XRD, Raman or whatever. Then the post costs would be negligable.
Cheers,
Joel
18th Sep 2017 15:18 UTCOwen Lewis
Eeeek!! What weight are you wanting to send for a charge like that? I'm very happy to determine accurate SGs from pieces down to 0.01 g. For a piece with a flat-polished surface and for determination of the RIs and other optical characteristics, the piece is best > 0.2 g and not more than 5g. What would be the cost to you of sending just a couple of such pieces to me in a padded envelope? The UK has a small packet rate fro such items. Mark the customs declaration 'Mineral specimens fro study' and give a nominal value equivalent to about GBP 5.00. Also, you can forget the return postage; I'll look after that for you, if you decide to proceed. You obviously try intelligently and are a quick learner with a good feel for what you are doing. On what you have contributed so far in this thread, I'm happy to encourage your study in this small way..

18th Sep 2017 17:49 UTCRio Nevin
On the shipping fee estimate website, I put a weight estimate of 20 grams (since 2.71 gram excludes envelope etc), and those rates were the output. So I tried again to put 5 grams on the website.. still the same insane rate.
Tomorrow morning I'll visit the post office to ask if there's a cheaper rate...
Thank you very much Owen.
Update:
I have visited my town's central post office. They actually provide a very cheap rate for slower deliveries, but they demand an MSDS accompanying my sample. I guess they're afraid that my sample will make people go mad or go boom... lol
Update:
After reading some posts about problems regarding customs and general unfamiliarity with "minerals",etc here in Mindat, maybe the officials I met thought that the "mineral" was some kind of consumables or chemicals, which explains why they asked for MSDS. Furthermore, Indonesian regulations classifying every item sent abroad as "export" does not make things easier.
Gotta try the smaller post office branch and send it there as a
19th Sep 2017 17:08 UTCPeter Slootweg 🌟

19th Sep 2017 17:40 UTCRio Nevin
The fracture of these pieces are not straight, as the ones I had observed on serpentine (also local material), either fracture or cleavage, were more straight. I only have 2 different pieces of serpentine so this may be not representative.
But on the bulk material, the fractures look like the ones in serpentine.
The pieces were all dry and not oiled when photographed, so, yeah, they seemed to be a bit too tranparent for nephrite. The thin flake shown in greenman3.jpg, however, was only 0.4-0.7mm thin. I have no prior experience with nephrite so I do not know whether nephrite is typically transparent at this thickness.
About the bruise, I don't think it gets bruised that easily, the piece fell down from my desk and touched the floor at that exact corner, so the whole momentum was put there. The rest was okay, which to me would be rather surprising if it were serpentine (considering how easy it broke when cut?)
Anyways, let's wait for Owen's result! It may take a while to get to Owen though as I chose the cheapest shipping. (It was 10-15 times cheaper than the faster services)
19th Sep 2017 18:38 UTCOwen Lewis

20th Sep 2017 01:59 UTCDoug Daniels

20th Sep 2017 02:36 UTCDonald B Peck Expert

27th Oct 2017 16:23 UTCRio Nevin
Owen e-mailed me on Sep 29th that he had received the sample.

29th Oct 2017 01:57 UTCTheodore Hansen Theo
1st Nov 2017 03:16 UTCOwen Lewis
Two of the pieces were quite thin flakes, the thinnest being only 0.68mm (max) in thickness. These show clearly irregularly shaped grains in the host material.
Weights were (ct):10.578, 0.631. 0.496 and 0.143
SG (in same order) 2.97, 2.97, 2.91, 2.72
Hardness (Mohs) 5.5, 5.5, 5.0, 5.0
Optical reaction to linearly polarised light. AGG (all)
UV LW & SW. Inert (all)
Fracture uneven.
Cleavage. None
Lustre waxy




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 19, 2024 20:30:57
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 19, 2024 20:30:57