Bernalite
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Bernalite
Formula:
Fe(OH)3 · nH2O (n = 0.0 to 0.25)
Colour:
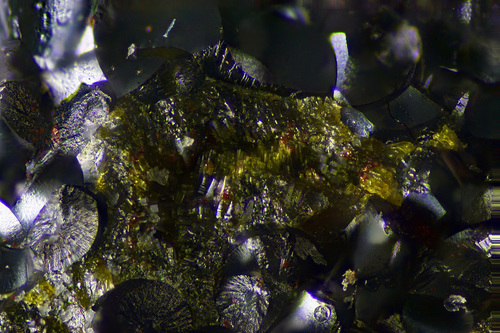
Dark bottle-green to yellow-green; yellowish bottle-green in thin section
Lustre:
Adamantine, Vitreous, Resinous
Hardness:
4
Specific Gravity:
3.32
Crystal System:
Orthorhombic
Name:
Named in honor of John Desmond Bernal (10 May 1901, Nenagh, County Tipperary, Ireland - 15 September 1971, London, England), eminent crystallographer and historian of science. He pioneered the use of X-ray crystallography in molecular biology and determined the structure of graphite. He also investigated the crystal chemistry of iron oxides and hydroxides.
Söhngeite Group.
A highly unusual, pseudo-cubic, bottle-green iron hydroxide. In bernalite, the iron containing octahedra units only share corners, while other iron hydroxides share both corners and edges, resulting in Fe-O distances in bernalite that are more consistent than other iron hydroxides. This causes a low crystal field stabilization energy which results in a green color as compared to the red yellow of other iron hydroxides.
A highly unusual, pseudo-cubic, bottle-green iron hydroxide. In bernalite, the iron containing octahedra units only share corners, while other iron hydroxides share both corners and edges, resulting in Fe-O distances in bernalite that are more consistent than other iron hydroxides. This causes a low crystal field stabilization energy which results in a green color as compared to the red yellow of other iron hydroxides.
Unique Identifiers
Mindat ID:
635
Long-form identifier:
mindat:1:1:635:6
GUID
(UUID V4):
(UUID V4):
8c57f88f-f743-4728-88e5-01b8d598740e
IMA Classification of Bernalite
Approved
IMA Formula:
Fe(OH)3
First published:
1992
Classification of Bernalite
4.FC.05
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
F : Hydroxides (without V or U)
C : Hydroxides with OH, without H2O; corner-sharing octahedra
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
F : Hydroxides (without V or U)
C : Hydroxides with OH, without H2O; corner-sharing octahedra
6.3.5.3
6 : HYDROXIDES AND OXIDES CONTAINING HYDROXYL
3 : X(OH)3
6 : HYDROXIDES AND OXIDES CONTAINING HYDROXYL
3 : X(OH)3
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Bnl | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Bernalite
Adamantine, Vitreous, Resinous
Transparency:
Transparent, Opaque
Colour:
Dark bottle-green to yellow-green; yellowish bottle-green in thin section
Streak:
Apple-green
Hardness:
4 on Mohs scale
Tenacity:
Brittle
Cleavage:
None Observed
Fracture:
Irregular/Uneven, Conchoidal
Density:
3.32 g/cm3 (Measured) 3.35 g/cm3 (Calculated)
Optical Data of Bernalite
Type:
Biaxial
RI values:
n = 1.92 - 1.94
Max Birefringence:
δ = 0.000

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Very High
Dispersion:
r > v, strong
Chemistry of Bernalite
Mindat Formula:
Fe(OH)3 · nH2O (n = 0.0 to 0.25)
Elements listed:
Common Impurities:
C,Pb,Si,Zn
Crystallography of Bernalite
Crystal System:
Orthorhombic
Class (H-M):
mmm (2/m 2/m 2/m) - Dipyramidal
Space Group:
Pmmn
Cell Parameters:
a = 7.544 Å, b = 7.56 Å, c = 7.558 Å
Ratio:
a:b:c = 0.998 : 1 : 1
Unit Cell V:
431.05 ų (Calculated from Unit Cell)
Z:
8
Morphology:
Flattened pyramidal crystals, pseudo-octahedral to pseudo-cubic, with slightly concave faces; also skeletal aggregates.
Twinning:
Polysynthetic, crosshatched, observed in thin section, probably pinacoidal.
Comment:
Pseudocubic. Originally described with space group Immm.
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0001607 | Bernalite | Birch W D, Pring A, Reller A, Schmalle H W (1993) Bernalite, Fe(OH)3, a new mineral from Broken Hill, New South Wales: Description and structure American Mineralogist 78 827-834 |  | 1993 | Broken Hill, New South Wales, Australia | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 3.784 Å | (100) |
| 1.692 Å | (17) |
| 2.393 Å | (16) |
| 2.676 Å | (15) |
| 1.892 Å | (10) |
| 1.545 Å | (9) |
| 2.023 Å | (6) |
Comments:
Recorded on type material
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| High-𝑇 alteration and/or metamorphism | |
| 32 : Ba/Mn/Pb/Zn deposits, including metamorphic deposits | |
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 55 : Anthropogenic mine minerals |
Type Occurrence of Bernalite
General Appearance of Type Material:
Flattened pyramidal crystals and pseudo-octahedra, to 3 mm.
Place of Conservation of Type Material:
Museum of Victoria, Melbourne, Australia;
South Australian Museum, Adelaide, Australia (No. G17627)
South Australian Museum, Adelaide, Australia (No. G17627)
Geological Setting of Type Material:
On a museum specimen from a metamorphosed Pb-Zn deposit, probably from the surface oxidation zone
Associated Minerals at Type Locality:
Reference:
Birch, W. D., Pring, A., Reller, A. and Schmalle, H. W. (1992) Bernalite: a new ferric hydroxide with perovskite structure. Naturwissenschaften: 79: 509-511.
Synonyms of Bernalite
Other Language Names for Bernalite
Relationship of Bernalite to other Species
Other Members of this group:
| Dzhalindite | In(OH)3 | Iso. m3 (2/m 3) : Im3 |
| Söhngeite | Ga(OH)3 | Tet. |
Common Associates
Associated Minerals Based on Photo Data:
Related Minerals - Strunz-mindat Grouping
| 4.FC.05 | Dzhalindite | In(OH)3 |
| 4.FC.05 | Söhngeite | Ga(OH)3 |
| 4.FC.10 | Burtite | Ca[Sn(OH)6] |
| 4.FC.10 | Mushistonite | (Cu,Zn,Fe2+)[Sn(OH)6] |
| 4.FC.10 | Natanite | Fe2+[Sn(OH)6] |
| 4.FC.10 | Schoenfliesite | Mg[Sn(OH)6] |
| 4.FC.10 | Vismirnovite | Zn[Sn(OH)6] |
| 4.FC.10 | Wickmanite | Mn2+[Sn(OH)6] |
| 4.FC.15 | Jeanbandyite | Fe3+xFe2+1-xSn(OH)6-xOx |
| 4.FC.15 | Mopungite | Na[Sb5+(OH)6] |
| 4.FC.15 | Stottite | Fe2+[Ge4+(OH)6] |
| 4.FC.15 | Tetrawickmanite | Mn2+[Sn4+(OH)6] |
| 4.FC.20 | Ferronigerite-2N1S | (Al,Fe,Zn)2(Al,Sn)6O11(OH) |
| 4.FC.20 | Magnesionigerite-6N6S | (Mg,Al,Zn)3(Al,Sn,Fe)8O15(OH) |
| 4.FC.20 | Magnesionigerite-2N1S | (Mg,Al,Zn)2(Al,Sn)6O11(OH) |
| 4.FC.20 | Ferronigerite-6N6S | (Al,Fe,Zn)3(Al,Sn,Fe)8O15(OH) |
| 4.FC.20 | Zinconigerite-2N1S | (Zn,Al,Mg)2(Al,Sn)6O11(OH) |
| 4.FC.20 | Zinconigerite-6N6S | Zn3Sn2Al16O30(OH)2 |
| 4.FC.25 | Magnesiotaaffeite-6N’3S | Mg2BeAl6O12 |
| 4.FC.25 | Magnesiotaaffeite-2N’2S | Mg3Al8BeO16 |
| 4.FC.25 | Ferrotaaffeite-2N’2S | Be(Fe,Mg,Zn)3Al8O16 |
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Industrial Uses:
None
Internet Links for Bernalite
mindat.org URL:
https://www.mindat.org/min-635.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Bernalite
Reference List:
Birch, W. D., Pring, A., Reller, A., Schmalle, H. (1992) Bernalite : a new ferric hydroxide with perovskite structure. Naturwissenschaften, 79 (11). 509-511 doi:10.1007/bf01135768
Birch, William D., Pring, Allan, Reller, Armin, Schmalle, Helmut W. (1993) Bernalite, Fe(OH)3, a new mineral from Broken Hill, New South Wales: Description and structure. American Mineralogist, 78 (7-8) 827-834
Localities for Bernalite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Australia (TL) | |
| Naturwiss. (1992) |
| R&M. 71:160-161 (1996) +1 other reference | |
| Parbhakar-Fox (2016) |
| Parbhakar-Fox (2016) | |
Germany | |
| Walenta (1992) +3 other references |
| Lapis 33 (10) |
| Thalheim et al. (2006) |
Iran | |
| Khorasanipour (2015) |
Italy | |
| Dott. Cristina Carbone-Dipteris-Genova: analysis June 2007 (paper in preparation) +1 other reference |
Japan | |
| Nagashima et al. (2016) |
Mexico | |
| Yta et al. (2005) |
Russia | |
| Sharygin +9 other references |
Spain | |
| Shuster et al. (2017) |
USA | |
| Pxrd and eds by Tony Kampf. Collected ... |
Quick NavTopAbout BernaliteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Clara Mine, Oberwolfach, Ortenaukreis, Freiburg Region, Baden-Württemberg, Germany