Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
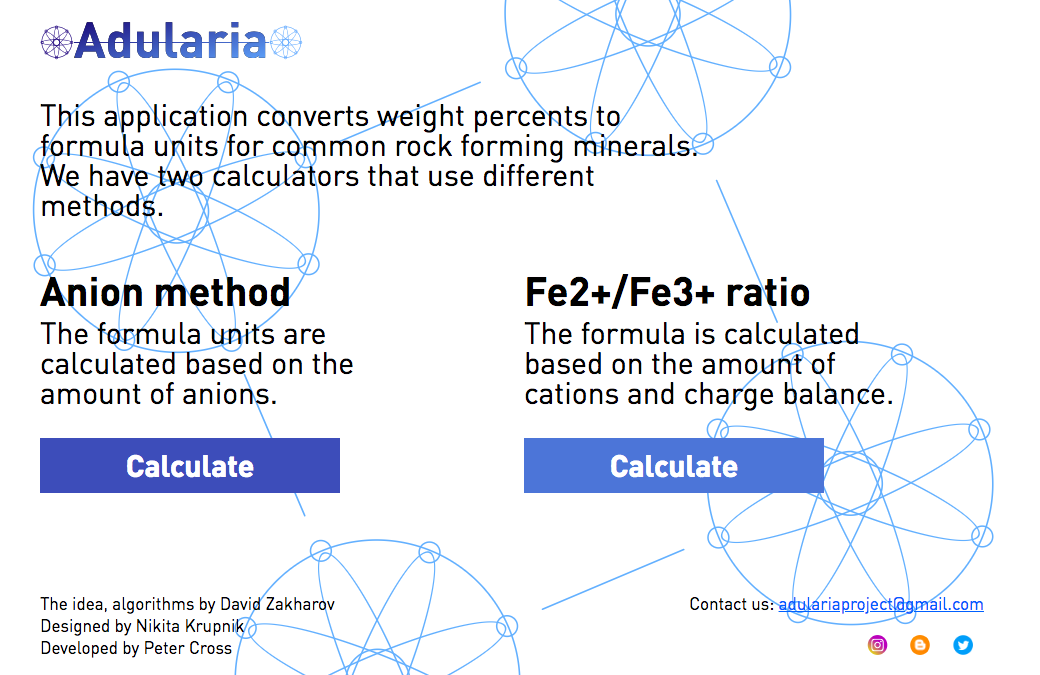
EducationMineral formula calculation application ADULARIA

21st Dec 2018 15:14 UTCDavid Zakharov
we made this little web-based application that conveniently re-calculates mineral formulas for selected common rock-forming minerals (feldspars, olivines, pyroxenes, etc.). We also have hydrous amphiboles and micas. ALSO, the calculator has the Fe2+/Fe3+ ratio algorithm implemented for pyroxenes, garnets and amphiboles. Please let me know if you would find this useful. We made it mostly for undergraduate students, but could be useful for researchers.
Link at https://adularia.app/
Best,
David
EDIT: the site moved to .app domain
21st Dec 2018 19:19 UTCFrank K. Mazdab 🌟 Manager
While the idea of having something like this is OK, I wonder how the complexity of some mineral normalizations, even for common minerals like the amphiboles, is accommodated by your app. Amphiboles, in fact, provide a great example.
In addition to an anion-based normalization scheme (which, incidentally, isn't easily adapted to samples that fall between the hydrous and anhydrous ends), there are actually six end-member cation-based normalization schemes for amphibole... these are:
1) only Si in the 8 IVT site (which forces all Al to be accommodated in the VIM sites)
2) all Si+Al in the 8 IVT sites (in this case there can be no Al in the VIM sites)
3) all Si+Al+Fe+Mg+Mn+Ti in the combined 13 IVT+VIM sites (in this case there can be no Mg+Fe+Mn in the VIIIM site)
4) all Si+Al+Fe+Mg+Mn+Ti+Ca in the combined 15 IVT+VIM+VIIIM sites (this forces all Na to be accommodated in the XIIA site)
5) all Si+Al+Fe+Mg+Mn+Ti+Ca+Na in the combined 15 IVT+VIM+VIIIM sites (this excludes any Na from being accommodated in the XIIA site)
6) all cations in the full 16 sites (this excludes vacancies in any site, including the XIIA site).
Note that I'm excluding the less common Li-bearing amphiboles here, so if one includes those, a couple of additional end-member cases can be envisioned that would either force all Li into VIM, all Li into VIIIM, or allow Li to distribute between both sites.
Some amphiboles will only work with one of these, while others can be normalized by several of these, but nonetheless each one will give a slightly different formula and will also give a different Fe3+/∑Fe ratio. For amphiboles that will normalize using multiple schemes, picking the best one involves assessing the calculated Fe3+/∑Fe ratio, the site fillings and the total, and optimizing among these. This isn't difficult to do (in a program like Excel), but in a "black-box" style app, how does the user assess this?
Numerous authors (commonly those with a geology major and a computing science minor) have put out papers in the past touting these canned approaches to normalizing minerals. I always wonder why?
My suggestion has always been that it's easier and a much better learning experience for a student, and certainly for a researcher, to spend half an hour putting a simple normalization scheme together in Excel. Once one is done for a complex case like amphibole, it's easy to copy that sheet and then adapt the copy to other simpler minerals like pyroxene, garnet, etc. I've put together a YouTube video on how you build one of these normalization routines, using epidote as an example:
https://www.youtube.com/watch?v=IbMLngyz4Cs&feature=youtu.be
Admittedly, this video is more than half an hour long, but the featured normalization includes some bells and whistles not necessary for a more simple approach to the problem. I'm thinking about making another more basic example. For those who don't like sitting through a video, I also have a more general written-out instruction sheet: https://www.rockptx.com/wp-content/uploads/2015/04/setting-up-mineral-normalizations-in-Excel.pdf
Frank

5th May 2019 21:50 UTCDavid Zakharov
firstly, thank you for such a detailed response. I am wowed by your efforts and videos. I am watching them now and will certainly use in the future. Your tutorials provide fundamental, detailed and precise instructions.
The idea behind out project was to have a user-friendly app that would deal with simple and user-friendly minerals like olivines and feldspars. We implemented anion-based schemes for fully hydrous and fully anhydrous amphiboles too just because it was easy. But they do not provide calculations for partially hydrous amphiboles. Distribution of Al across M and T position is up to humans. The calculator spits out total units and the user needs to write down the formula and place elements according to the availability and vacancies in the formula.
Researchers and students indeed must do their own work on paper, Excel spreadsheets and program scripts to perform their calculations.
As for Fe/Fe ratios, the second button (https://adularia.org/ferrum/ferrumjq.html) features the method described in Droop, 1987 (Min.Mag.), where the amount of ferric iron is derived from charge balance given the fixed amount of anions and cations per anhydrous formula. Amphiboles are more complex than other minerals, we implemented the simple scheme, where 13 is the number for cation basis and 23 for anion basis. It works for Ca-amphiboles, where the first position in the formula is vacant.
Again, thank you for the feedback, I will think more about how to improve our calculator.
best,
david
5th May 2019 22:08 UTCFrank K. Mazdab 🌟 Manager
Actually, since the original start of this thread back in December, when I wrote, "I'm thinking about making another more basic example"... well, I got off my butt and actually did it, and indeed my simplified video series specifically describes setting up amphibole normalizations in Excel. You may have already stumbled across it, but if not, the playlist (it's a set of 8 shorter videos instead of one long grueling one) is at:
https://www.youtube.com/watch?v=GoPhdGAT3AY&list=PLOH52vpmy19lFSP2qoa9Ckeycf8TCPkbB

6th May 2019 00:51 UTCDavid Zakharov
I saw that! Thanks! I will be using it next time I TA/teach mineralogy.
Best regards,
David




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 26, 2024 02:52:46
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 26, 2024 02:52:46