Home PageAbout MindatThe Mindat ManualHistory of MindatCopyright StatusWho We AreContact UsAdvertise on Mindat
Donate to MindatCorporate SponsorshipSponsor a PageSponsored PagesMindat AdvertisersAdvertise on Mindat
Learning CenterWhat is a mineral?The most common minerals on earthInformation for EducatorsMindat ArticlesThe ElementsThe Rock H. Currier Digital LibraryGeologic Time
Minerals by PropertiesMinerals by ChemistryAdvanced Locality SearchRandom MineralRandom LocalitySearch by minIDLocalities Near MeSearch ArticlesSearch GlossaryMore Search Options
The Mindat ManualAdd a New PhotoRate PhotosLocality Edit ReportCoordinate Completion ReportAdd Glossary Item
Mining CompaniesStatisticsUsersMineral MuseumsClubs & OrganizationsMineral Shows & EventsThe Mindat DirectoryDevice SettingsThe Mineral Quiz
Photo SearchPhoto GalleriesSearch by ColorNew Photos TodayNew Photos YesterdayMembers' Photo GalleriesPast Photo of the Day GalleryPhotography
╳Discussions
💬 Home🔎 Search📅 LatestGroups
EducationOpen discussion area.Fakes & FraudsOpen discussion area.Field CollectingOpen discussion area.FossilsOpen discussion area.Gems and GemologyOpen discussion area.GeneralOpen discussion area.How to ContributeOpen discussion area.Identity HelpOpen discussion area.Improving Mindat.orgOpen discussion area.LocalitiesOpen discussion area.Lost and Stolen SpecimensOpen discussion area.MarketplaceOpen discussion area.MeteoritesOpen discussion area.Mindat ProductsOpen discussion area.Mineral ExchangesOpen discussion area.Mineral PhotographyOpen discussion area.Mineral ShowsOpen discussion area.Mineralogical ClassificationOpen discussion area.Mineralogy CourseOpen discussion area.MineralsOpen discussion area.Minerals and MuseumsOpen discussion area.PhotosOpen discussion area.Techniques for CollectorsOpen discussion area.The Rock H. Currier Digital LibraryOpen discussion area.UV MineralsOpen discussion area.Recent Images in Discussions
Identity HelpMadagascar Mini-Monster

16th Mar 2009 15:38 UTCRudy Bolona Expert
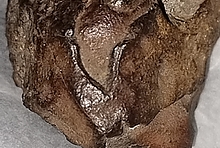
I ordered this specimen from a Canadian dealer a couple of years ago. He had it listed as Monazite. When I received it I knew it wasn't monazite. The color, specific gravity, radioactivity, and crystal form were off. I posted it on Mindat, and a well known mineralogist said it was cyrtolite. It was in the cyrtolite gallery in Mindat for awhile. The more I looked at it, the more doubts I had it was cyrtolite. No cyrtolite is as radioactive as this specimen. 12000 CPM on my digital geiger counter. I took it to the Colorado School of Mines this past weekend. A student there working on his doctorate has access to an x-ray fluorescence gun. He tested it. No zirconium was present. He did find these elements: titanium, niobium, tantalum, iron, lead, uranium, and even some arsenic. Titanium created the highest peak. The amount of lead was a bit higher than the uranium. Possibly a plumbobetafite? This is my most bizarre specimen. No specific mine locale was on the label. Any suggestions out there?
Thanks,
16th Mar 2009 17:09 UTCJohan Kjellman Expert
how big is it? Can you relate the counts to some known samples of similar size. do you have any fresh surfaces exposed? You have made a good guess but we really need an analysis. There is an old literature creature called plumboniobite which could be something similar, a uranopolycrase (ideally UTi2O8) with a large amount of the U transformed to Pb would also be a candidate. Also be ware that sometimes peaks and amounts are not corelated..
cheers
16th Mar 2009 17:33 UTCChris Stefano Expert

16th Mar 2009 17:49 UTCJohn Duck
16th Mar 2009 17:58 UTCChris Stefano Expert

16th Mar 2009 22:01 UTCRudy Bolona Expert
The specimen is 7 x 4 x 3.5 cm. The interior is a resinous to glassy golden brown. Euxenite, polycrase, samarskite, fergusonite, etc.. usually have a much darker to black interior. The specific gravity of this specimen is 4.0, the previous minerals listed are denser starting at around 4.5 and up. Hydration could be a factor though. The crystal cross section looks triangular. Maybe an old samiresite? Here are more photos showing these aspects. I need to have a professional analysis done. Which won't damage it. Right now, to me this thing is one of a kind. But who? The specimen is two crystals that are opposed and in contact. ::o
Thanks
16th Mar 2009 22:41 UTCTomasz Praszkier Manager
I analysed several similar specimens to this one - usually this are fergusonite or monazite. It is surely not betafite in my opinion.
This specimen come from Ambatofotsy?
Tom

16th Mar 2009 23:05 UTCRudy Bolona Expert
By the way thank you for the very nice pezzotaite. The specimen came only labeled as Madagascar, no specific mine was listed. The analysis showed no phosphorus or cerium, so it can't be a monazite. Perhaps you are right on the fergusonite. The x-ray fluorescence gun was not able to determine amounts of niobium, and tantalum, only their presence. Titanium came up significantly. strange crystal shape for this piece.
Thank You
Rudy
17th Mar 2009 00:14 UTCTomasz Praszkier Manager
Tom
17th Mar 2009 02:36 UTCPavel Kartashov Manager
I am think this is mixture of high hydrated pyrochlore group minerals after unknown precursor - i.e. samiresite according to elevated Pb content. May be from Samiresi.
Mineral can't to be plumbopyrochlore, plumbomicrolite and especially plumbobetafite from most common considerations.
17th Mar 2009 02:45 UTCMatt Neuzil Expert

17th Mar 2009 03:10 UTCRudy Bolona Expert
I tend to agree with you, that this is a pyrochlore group mineral that has undergone transmogrification. I will have this professionally analyzed one day. For now it is known as triple M, ( Madagascar Mini- Monster ). :P
17th Mar 2009 03:20 UTCPavel Kartashov Manager
18th Mar 2009 09:34 UTCSebastian Möller Expert
An XRD (X-ray powder diffraction) analysis would give you at least a hint on the group it belongs to and the structure, but you will have to powder a little piece of the sample.
Regarding the lead: Could be normal, non radiogenic lead or radiogenic lead due to decay of uranium. With the rocks in madagascar being relatively old (about 1 billion years to 500 million years) an amount of lead derived from radioactive decay could be there. But it will not explain all the lead as most of the uranium in natural samples is U-238 (99,3 % of total natural U) which has a half life of 4,5 billion years, only 0,7 % being U-235 with a half life around 750 million years. So a maximum of a half of the U-235 and less than 0.25x0.5 of the U-238 will have decayed. That can, by no means, make a lead peak higher than a uranium peak if there isn't some non-radiogenic lead present.
Regards,
Sebastian Möller
18th Mar 2009 10:04 UTCJohan Kjellman Expert
be wary of qualitative spectra with assigned elemental peaks.
I get a thorite "feeling" on your sample and there is a possiblity for "qualitative mixup" of Ta for Si and Ti for Th peaks
would explain the high activity
also from my experience thorite is very common in NYF pegs

18th Mar 2009 13:00 UTCRudy Bolona Expert
Sebastian: All the lead can't be from decay. You are right the uranium takes so long to break down.
Johan: I also thought thorite.
Guess what? I broke down and removed a small chip and have sent it out for a good analysis. When I get the results. I will post them on Mindat.
Cheers!
18th Mar 2009 15:42 UTCPavel Kartashov Manager
Th peaks on EDS spectre are quite far from Ti peaks, it is impossible to confuse them.
Ta peak really significantly overlap Si one, but besides that Ta has many small peaks at the right part of spectra.
Dear Sebastian,
I am more than 99.5 % assured, that this mineral is metamict. So its PXRD has some sence only after heating. After heating of such specimens 4-5 new formed phases can to appear. By the way this is the reason, why 'samiresite' hadn't became individual mineral.
Of course main part of lead in such niobates are stable, nonradiogen isotopes. On late stages of formation such pegmatites lead concentration can increase significantly with formation of abundant galena. But now it mainly altered into cerussite/anglesite/sulphur agregates. Such alteration of galena may be source of very late saturation of pyrochlore group minerals with lead from ground waters. Pyrochlores are very similar to zeolitis in sence their high cation-exchange ability.




Mindat.org is an outreach project of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit organization.
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 29, 2024 16:25:12
Copyright © mindat.org and the Hudson Institute of Mineralogy 1993-2024, except where stated. Most political location boundaries are © OpenStreetMap contributors. Mindat.org relies on the contributions of thousands of members and supporters. Founded in 2000 by Jolyon Ralph.
Privacy Policy - Terms & Conditions - Contact Us / DMCA issues - Report a bug/vulnerability Current server date and time: April 29, 2024 16:25:12