Mazzite-Na
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Mazzite-Na
Formula:
Na8[Al4Si14O36]2 · 30H2O
Colour:
White
Lustre:
Vitreous
Specific Gravity:
2.16
Crystal System:
Hexagonal
Member of:
Name:
Named for its relationship to mazzite-Mg, with a suffix indicating the incorporation of sodium rather than magnesium.
The root name is for Fiorenzo Mazzi (1924 Forence, Italy - 25 September 2017, Pavia, Italy), Professor of Mineralogy at the University of Pavia, Italy. He made significant contributions to the understanding of zeolite structure.
The root name is for Fiorenzo Mazzi (1924 Forence, Italy - 25 September 2017, Pavia, Italy), Professor of Mineralogy at the University of Pavia, Italy. He made significant contributions to the understanding of zeolite structure.
Unique Identifiers
Mindat ID:
27399
Long-form identifier:
mindat:1:1:27399:4
GUID
(UUID V4):
(UUID V4):
e56f849a-100c-42aa-8c66-b3b86bcb49d7
Classification of Mazzite-Na
Approved
Approval year:
2003
First published:
2005
9.GC.20
9 : SILICATES (Germanates)
G : Tektosilicates with zeolitic H2O; zeolite family
C : Chains of doubly-connected 4-membered rings
9 : SILICATES (Germanates)
G : Tektosilicates with zeolitic H2O; zeolite family
C : Chains of doubly-connected 4-membered rings
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Maz-Na | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Mazzite-Na
Vitreous
Transparency:
Transparent
Colour:
White
Streak:
White
Hardness Data:
Could not be measured
Tenacity:
Flexible
Cleavage:
None Observed
Density:
2.16 g/cm3 (Measured) 2.18 g/cm3 (Calculated)
Optical Data of Mazzite-Na
Type:
Uniaxial (+)
RI values:
nα = 1.472 nγ = 1.471
Max Birefringence:
δ = 0.001

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Moderate
Chemical Properties of Mazzite-Na
Formula:
Na8[Al4Si14O36]2 · 30H2O
IMA Formula:
Na8(Si28Al8)O72 · 30H2O
Crystallography of Mazzite-Na
Crystal System:
Hexagonal
Class (H-M):
6/mmm (6/m 2/m 2/m) - Dihexagonal Dipyramidal
Space Group:
P63/mmc
Cell Parameters:
a = 18.2343 Å, c = 7.6371 Å
Ratio:
a:c = 1 : 0.419
Unit Cell V:
2,199.06 ų (Calculated from Unit Cell)
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0003819 | Mazzite-Na | Arletti R, Galli E, Vezzalini G, Wise W S (2005) Mazzite-Na, a new zeolite from Boron, California: Its description and crystal structure American Mineralogist 90 1186-1191 |  | 2005 | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 9.08 Å | (100) |
| 6.86 Å | (70) |
| 5.95 Å | (70) |
| 4.681 Å | (40) |
| 3.787 Å | (80) |
| 3.511 Å | (40) |
| 3.150 Å | (70) |
Comments:
From the type description.
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 3a: Earth’s earliest Hadean crust | >4.50 |
| 10 : Basalt-hosted zeolite minerals |
Type Occurrence of Mazzite-Na
General Appearance of Type Material:
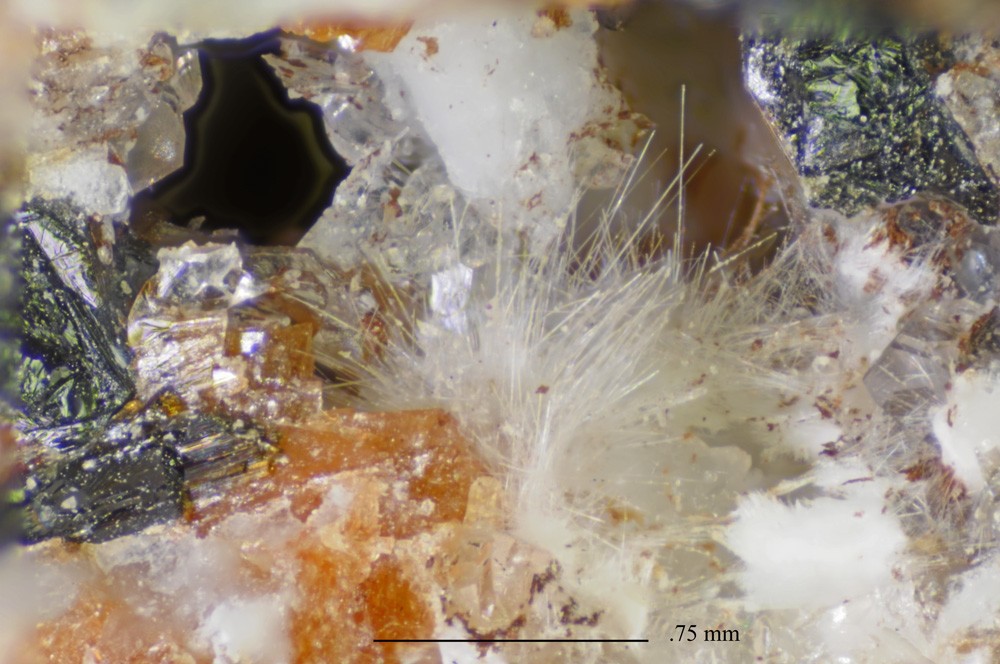
White, very thin, flexible fibers up to 2 mm in length, commonly filling small cavities as satiny mats.
Place of Conservation of Type Material:
n.d.
Geological Setting of Type Material:
In basalt.
Associated Minerals at Type Locality:
Synonyms of Mazzite-Na
Other Language Names for Mazzite-Na
Relationship of Mazzite-Na to other Species
Member of:
Other Members of this group:
| Mazzite-Mg | (Mg,K,Ca)5(Si26Al10)O72 · 28H2O | Hex. 6/mmm (6/m 2/m 2/m) : P63/mmc |
Common Associates
Associated Minerals Based on Photo Data:
Related Minerals - Strunz-mindat Grouping
| 9.GC. | Garronite-Na | Na6(Al6Si10O32) · 8.5H2O | Mon. 2 |
| 9.GC. | Gismondine-Sr | Sr4(Si8Al8O32) · 9H2O | Orth. 2 2 2 : C2 2 21 |
| 9.GC.05 | Amicite | K2Na2Al4Si4O16 · 5H2O | Mon. 2 |
| 9.GC.05 | Garronite-Ca | Na2Ca5Al12Si20O64 · 27H2O | Tet. 4 2m : I4m2 |
| 9.GC.05 | Gismondine-Ca | CaAl2Si2O8 · 4H2O | Mon. 2/m : P21/b |
| 9.GC.05 | Gobbinsite | Na5(Si11Al5)O32 · 11H2O | Orth. mmm (2/m 2/m 2/m) : Pnma |
| 9.GC.05 | Gismondine-Ba | Ba2Al4Si4O16 · 4-6H2O | |
| 9.GC.10 | Harmotome | Ba2(Si12Al4)O32 · 12H2O | Mon. 2/m : P21/m |
| 9.GC.10 | Phillipsite-Ca | (Ca0.5,K,Na,Ba0.5)4-7[Al4-7Si12-9O32] . 12H2O | Mon. 2/m : P21/m |
| 9.GC.10 | Phillipsite-K | (K,Na,Ca0.5,Ba0.5)4-7[Al4-7Si12-9O32] . 12H2O | Mon. 2/m : P21/m |
| 9.GC.10 | Phillipsite-Na | (Na,K,Ca0.5,Ba0.5)4-7[Al4-7Si12-9O32] · 12H2O | Mon. 2/m : P21/m |
| 9.GC.10 | Flörkeite | (K3Ca2Na)[Al8Si8O32] · 12H2O | Tric. 1 : P1 |
| 9.GC.10 | Martinandresite | Ba2(Al4Si12O32) · 10H2O | Orth. mmm (2/m 2/m 2/m) : Pmmn |
| 9.GC.15 | Merlinoite | (K,Na)5(Ca,Ba)2Al9Si23O64 · 23H2O | Orth. mmm (2/m 2/m 2/m) : Immm |
| 9.GC.20 | Mazzite-Mg | (Mg,K,Ca)5(Si26Al10)O72 · 28H2O | Hex. 6/mmm (6/m 2/m 2/m) : P63/mmc |
| 9.GC.25 | Perlialite | K9Na(Ca,Sr)[Al2Si4O12]6 · 15H2O | Hex. 6/mmm (6/m 2/m 2/m) : P6/mmm |
| 9.GC.30 | Boggsite | Ca8Na3(Si,Al)96O192 · 70H2O | Orth. mmm (2/m 2/m 2/m) : Imma |
| 9.GC.35 | Paulingite-Ca | (Ca,K,Na,Ba, ◻)10 (Si, Al)42O84 · 34H2O | Iso. m3m (4/m 3 2/m) |
| 9.GC.35 | Paulingite-K | (K2,Ca,Na2,Ba)5[Al10Si35O90] · 45H2O | Iso. m3m (4/m 3 2/m) |
| 9.GC.35 | Paulingite-Na | (Na2,K2,Ca,Ba)5[Al10Si35O90] · 45H2O | Iso. |
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Mazzite-Na
mindat.org URL:
https://www.mindat.org/min-27399.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Mazzite-Na
Reference List:
Localities for Mazzite-Na
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Canada | |
| |
USA (TL) | |
|
Quick NavTopAbout Mazzite-NaUnique IdentifiersClassification Mineral SymbolsPhysical Properties Optical Data Chemical Properties Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 




Poudrette quarry, Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada