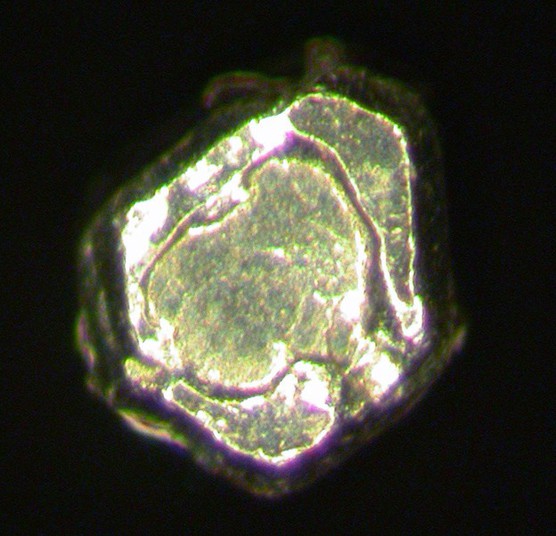
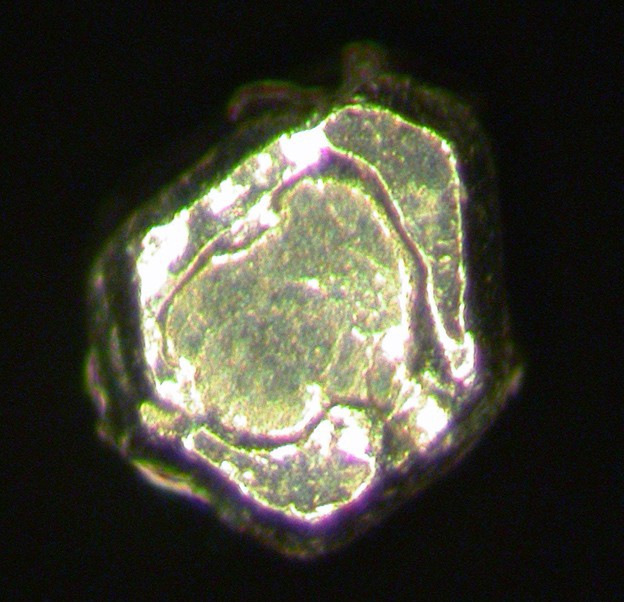
Osmium
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Osmium
Formula:
(Os,Ir,Ru)
Colour:
White
Lustre:
Metallic
Hardness:
6 - 7
Specific Gravity:
22.48
Crystal System:
Hexagonal
Name:
After the Greek for odor, for its pungent and irritating odor when heated in air.
Unique Identifiers
Mindat ID:
3037
Long-form identifier:
mindat:1:1:3037:9
GUID
(UUID V4):
(UUID V4):
80615b31-9b3a-4110-aa77-a033bd1ef187
IMA Classification of Osmium
Approved, 'Grandfathered' (first described prior to 1959)
IMA status notes:
Redefined by the IMA
IMA Formula:
Os
Approval history:
Redefined 1991 s.p.: Harris and Cabri (1991).
Classification of Osmium
1.AF.05
1 : ELEMENTS (Metals and intermetallic alloys; metalloids and nonmetals; carbides, silicides, nitrides, phosphides)
A : Metals and Intermetallic Alloys
F : Platinum group elements
1 : ELEMENTS (Metals and intermetallic alloys; metalloids and nonmetals; carbides, silicides, nitrides, phosphides)
A : Metals and Intermetallic Alloys
F : Platinum group elements
Dana 7th ed.:
1.2.2.1
1.2.2.1
1 : NATIVE ELEMENTS AND ALLOYS
2 : Platinum Group Metals and Alloys
1 : NATIVE ELEMENTS AND ALLOYS
2 : Platinum Group Metals and Alloys
1.76
1 : Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and Au)
1 : Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and Au)
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Os | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Os | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
Physical Properties of Osmium
Metallic
Transparency:
Opaque
Colour:
White
Streak:
Grey
Hardness:
6 - 7 on Mohs scale
Hardness:
VHN25=1206 - 1246 kg/mm2 - Vickers
Density:
22.48 g/cm3 (Measured) 22.59 g/cm3 (Calculated)
Optical Data of Osmium
Anisotropism:
Strong, reddish orange
Reflectivity:
| Wavelength | R1 | R2 |
|---|---|---|
| 420nm | 62.7% | 63.9% |
| 460nm | 63.9% | 64.6% |
| 500nm | 63.3% | 63.8% |
| 540nm | 62.1% | 62.7% |
| 580nm | 60.9% | 61.4% |
| 620nm | 59.9% | 60.2% |
| 660nm | 59.3% | 59.9% |
| 700nm | 59.4% | 60.0% |
Graph shows reflectance levels at different wavelengths (in nm). Top of box is 100%. Peak reflectance is 64.6%.
R1 shown in black, R2 shown in red
Colour in reflected light:
White with bluish gray tint
Pleochroism:
Weak
Chemistry of Osmium
Mindat Formula:
(Os,Ir,Ru)
Elements listed:
Common Impurities:
Pt,Rh,Pd
Crystallography of Osmium
Crystal System:
Hexagonal
Class (H-M):
6/mmm (6/m 2/m 2/m) - Dihexagonal Dipyramidal
Space Group:
P63/mmc
Cell Parameters:
a = 2.7341 Å, c = 4.3197 Å
Ratio:
a:c = 1 : 1.58
Unit Cell V:
27.96 ų (Calculated from Unit Cell)
Z:
2
Morphology:
Prismatic inclusions.
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0011188 | Osmium | Wyckoff R W G (1963) Second edition. Interscience Publishers, New York, New York Hexagonal closest packed, hcp, structure Crystal Structures 1 7-83 | 1963 | 0 | 293 | ||
| 0016737 | Osmium | Levy C, Picot P (1961) Nouvelles donnees sur les composes iridium-osmium. Existence de l'osmium natif _cod_database_code 1008870 Bulletin de la Societe Francaise de Mineralogie et de Cristallographie 84 312-317 | 1961 | 0 | 293 | ||
| 0020238 | Osmium | Mochalov A G, Dmitrenko G G, Rudashevsky N S, Zhernovsky I V, Boldyreva M M (1998) Hexaferrum (Fe,Ru),(Fe,Os),(Fe,Ir) - a new mineral Zapiski Vserossijskogo Mineralogicheskogo Obshchestva 127 41-51 | 1998 | synthetic | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 2.076 Å | (100) |
| 2.367 Å | (35) |
| 2.160 Å | (35) |
| 1.3668 Å | (20) |
| 1.2300 Å | (20) |
| 1.1551 Å | (20) |
| 1.595 Å | (18) |
Comments:
Synthetic
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Pre-terrestrial "Ur-minerals" | >4.57 |
| 1 : Stellar atmosphere condensates | |
| Stage 1: Primary nebular phases | 4.567-4.561 |
| 3 : Solar nebular condensates (CAIs, AOAs, URIs) | >4.565 |
| Near-surface Processes | |
| 26 : Hadean detrital minerals | |
| Stage 4b: Highly evolved igneous rocks | >3.0 |
| 36 : Carbonatites, kimberlites, and related igneous rocks | |
| 37 : Layered igneous intrusions and related PGE minerals | |
| Stage 5: Initiation of plate tectonics | <3.5-2.5 |
| 38 : Ophiolites |
Geological Setting:
From ultramafic rocks and placers.
Synonyms of Osmium
Platinum-Group Elements (in part)
Other Language Names for Osmium
Arabic:أوزميوم
Armenian:Օսմիում
Basque:Osmio
Belarusian:Осмій
Bengali:অসমিয়াম
Bosnian:Osmijum
Bulgarian:Осмий
Catalan:Osmi
Corsican:Osmiu
Croatian:Osmij
Czech:Osmium
Danish:Osmium
Dutch:Osmium
Esperanto:Osmio
Estonian:Osmium
Farsi/Persian:اسمیوم
Finnish:Osmium
French:Osmium
Friulian:Osmi
Galician:Osmio
Greek:Όσμιο
Hebrew:אוסמיום
Hungarian:Ozmium
Icelandic:Osmín
Ido:Osmio
Indonesian:Osmium
Italian:Osmio
Japanese:オスミウム
Javanese:Osmium
Korean:오스뮴
Kurdish (Latin Script):Osmiyûm
Latin:Osmium
Latvian:Osmijs
Lithuanian:Osmis
Lojban:Jinmrbosmi
Luxembourgish:Osmium
Malayalam:ഓസ്മിയം
Manx:Osmium
Norwegian:Osmium
Norwegian (Nynorsk):Osmium
Occitan:Òsmi
Polish:Osm
Portuguese:Ósmio
Romanian:Osmiu
Russian:Осмий
Serbian:Осмијум
Serbo-Croatian:Osmijum
Sicilian:Òsmiu
Simplified Chinese:自然锇
Slovak:Osmium
Slovenian:Osmij
Spanish:Osmio
Swedish:Osmium
Thai:ออสเมียม
Traditional Chinese:自然鋨
Turkish:Osmiyum
Ukrainian:Осмій
Varieties of Osmium
| Iridosmine | A variety of osmium rich in iridium (all natural osmium contains some iridium). |
| Rhodic Nevyanskite | A variety of Osmium containing Rhodium and Iridium. |
Common Associates
Associated Minerals Based on Photo Data:
| 11 photos of Osmium associated with Iridium | (Ir,Os,Ru) |
| 7 photos of Osmium associated with Ruthenium | (Ru,Ir) |
| 5 photos of Osmium associated with Isoferroplatinum | Pt3Fe |
| 5 photos of Osmium associated with Rutheniridosmine | (Ir,Os,Ru) |
| 5 photos of Osmium associated with Laurite | RuS2 |
| 4 photos of Osmium associated with Iridarsenite | (Ir,Ru)As2 |
| 4 photos of Osmium associated with Platinum | Pt |
| 3 photos of Osmium associated with Iridosmine | (Os,Ir) |
| 2 photos of Osmium associated with Iron-Platinum alloy | (Pt,Fe) |
| 2 photos of Osmium associated with Osmiridium | (Ir,Os,Ru) |
Related Minerals - Strunz-mindat Grouping
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Osmium
mindat.org URL:
https://www.mindat.org/min-3037.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Osmium
Reference List:
Harris, D. C., Cabri, L. J. (1973) The nomenclature of the natural alloys of osmium, iridium and ruthenium based on new compositional data of alloys from world-wide occurrences. The Canadian Mineralogist, 12 (2) 104-112
Localities for Osmium
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Albania | |
| www.env.duke.edu (n.d.) |
Australia | |
| Sydney Fawns (1905) |
| Carlo Tortorella | |
| Dana's System of Mineralogy | |
| Webb (2014) |
| Pavel.M. Kartashov (n.d.) |
| G. A. Albertson Data |
| G. A. Albertson Data |
| G. A. Albertson Data | |
| Carlo Tortorella | |
| Bottrill & Baker in prep |
| Bottrill +2 other references | |
| Bottrill et al. (2008) |
| Bottrill et al. (2008) |
| Bottrill et al. (2008) |
| Bottrill & Baker 2008 | |
| Bottrill et al. (2008) |
| Butt et al. (2003) |
| Simpson (1952) |
Austria | |
| Malitch et al. (2002) |
Brazil | |
| 33rd International Geological Congress (2008) |
| Garuti et al. (2012) |
Bulgaria | |
| Цинцов (1998) |
| Atanasov (1990) |
| Anthony et al. (1990) |
Canada | |
| Canadian Mineralogist (2005) |
| Harris et al. (1973) | |
| Anthony et al. (1990) |
| BC Government Mining Report - 1918 |
| Canadian Mineralogist (2005) |
| Peatfield (n.d.) |
| Harris et al. (1973) |
| Nixon et al. (1990) | |
| Harris et al. (1973) | |
| Nixon et al. (1990) | |
| Escayola et al. (2011) |
| Canadian Mineralogist 28 |
| J.J. O'Neill and H.C. Gunning et al. (Pg. 55) |
| Traill (1983) | |
| Traill (1983) | |
| Fedortchouk et al. (eds.) |
| Barkov et al. (2008) |
Chile | |
| González-Jiménez et al. (2016) |
| Galdames et al. (2010) | |
China | |
| Bilun Luo (1979) +1 other reference |
| Yu Zuxiang (1985) | |
| Yu Zuxiang (1998) | |
| Chen Lichang et al. (1994) |
| Chen Keqiao et al. (1981) |
| Dianhao Huang et al. (1995) | |
| Anthony et al. (1990) |
| Shi et al. (2006) |
| Guo et al. (2016) +1 other reference |
| Wenji Bai et al. (2004) |
| Jianxun Bai (1987) |
| Hercule Shen |
| Min Liu (2002) |
Colombia | |
| Min Mag (1997) |
Costa Rica | |
| Federica Zaccarini et al. (2010) |
Czech Republic | |
| Pasava et al. (2015) |
Ecuador | |
| Barron et al. (2024) |
| Weiser et al. (1993) |
Ethiopia | |
| Pavel.M. Kartashov (n.d.) |
| Kebede H. Belete +1 other reference |
| Stanley et al. (2005) | |
| Cabri et al. (1981) | |
Finland | |
| Törroos Ragnar +1 other reference | |
| Stanislav S. Gornostayev (2000) |
France | |
| Ducluzaux B. (2022) |
| Ducluzaux (2018) |
| Lorand et al. (2018) |
| Ducluzaux B. (2022) |
| Ducluzaux (2021) +1 other reference |
| Economic Geology: 89: 1454-1468 +1 other reference |
| Auge et al. (1995) |
| Y. Moëlo : "Compte-rendu de la ... +2 other references |
| Ducluzaux (2022) |
Germany | |
| Harald G. Dill et al. (2009) |
| Dill et al. (2021) | |
| Oberthür et al. (2016) |
Greece | |
| www.researchgate.net (n.d.) +1 other reference |
| Economou-Eliopoulos et al. (2019) |
| Argirios Kapsiotis et al. (2011) |
| Auge (1985) +1 other reference |
| Kapsiotis et al. (2006) | |
| Auge (1985) |
India | |
| Canadian Mineralogist 40:277-309 |
| Prichard et al. (2018) | |
Indonesia | |
| Frank Keutsch specimen +1 other reference |
Iran | |
| Jannessary et al. (2012) |
Japan | |
| Nishio-Hamane et al. (2018) |
| www.mineralmundi.com |
| Urashima et al (1972) +1 other reference | |
| Masutomi Museum specimen (Kyoto) |
| Robert C. Smith | |
| Suzuki (1953) | |
| NISHIO–HAMANE et al. (2022) |
| Dr Daisuke Nishio-Hamane +1 other reference | |
| NISHIO–HAMANE et al. (2022) | |
| NISHIO–HAMANE et al. (2022) |
| NISHIO–HAMANE et al. (2022) | |
| NISHIO–HAMANE et al. (2022) |
| NISHIO–HAMANE et al. (2022) |
| Dr Daisuke Nishio-Hamane +1 other reference |
| Sadanaga et al. (1974) |
| Mitsubishi Corporation mineral ... |
| Sadanaga et al. (1974) | |
| NISHIO–HAMANE et al. (2019) |
Kazakhstan | |
| doi.org (n.d.) +2 other references |
| Saveliev et al. (2023) |
| Saveliev et al. (2023) | |
Morocco | |
| S. Weiß: Lapis 31 (7/8) |
Myanmar | |
| Hagen et al. (1990) +1 other reference |
New Zealand | |
| ... |
Norway | |
| Econ Geol (1993) +1 other reference |
| Nilsson (1990) |
| Nilsson et al. (1998) |
Oman | |
| Ahmed et al. (2003) |
Papua New Guinea | |
| www.minport.com (n.d.) |
| Mineralogy and Petrology 68:159-176 (2000) |
| [AmMin 85:1325] +1 other reference |
Philippines | |
| Oberthür et al. (2017) |
Russia | |
| Nesterenko +4 other references |
| Stepanov et al. (2007) |
| Garuti et al. (2021) |
| Pavel M. Kartashov analytical data 2018 |
| Airiyants et al. (2020) |
| Kiseleva +3 other references |
| Airiyants et al. (2022) | |
| Pavel.M. Kartashov (n.d.) |
| Zaykov et al. (2017) | |
| Zaykov et al. (2017) | |
| Razin et al. (1989) |
| Gornostayev et al. (1999) |
| Pekov (1998) |
| Berdnikov et al. (2020) +1 other reference |
| Mandarino (2000) |
| Tolstykh et al. (2000) |
| Sidorov et al. (2021) |
| Sidorov et al. (2004) +3 other references |
| Vlasov et al. (2002) | |
| Tolstykh et al. (2004) +1 other reference | |
| Tolstykh et al. (2004) | |
| Cabri et al. (2022) | |
| Kutyrev et al. (2020) +1 other reference | |
| Kutyrev et al. (2018) | |
| Razin et al. (1981) |
| Подлипский et al. (1999) |
| Pekov (1998) +1 other reference | |
| Tolstykh et al. (2009) |
| Nesterenko +7 other references |
| Pekov (1998) +2 other references |
| Cabri et al. (1997) | |
| Lavrik et al. (2020) |
| Novgorodova et al. (1995) +1 other reference |
| ... | |
| Barkov et al. (2018) |
| Barkov et al. (2021) +1 other reference | |
| Malitch K.N. et al. (2001) +1 other reference |
| Pavel.M. Kartashov (n.d.) +1 other reference | |
| Merkle et al. (2012) | |
| Merkle et al. (2012) | |
| Malitch et al. (2022) |
| Malitch et al. (2022) | |
| Yurichev et al. (2021) |
| Barkov et al. (2019) +1 other reference |
| Barkov et al. (2018) | |
| Feklichev et al. (1998) | |
| Yury L. Voytekhovsky (2008) |
| Neradovsky et al. (2008) | |
| Barkov +4 other references |
| ROGULINA et al. (2004) |
| Shcheka G.G. et al. (2004) |
| [World of Stones 12:49] | |
| Pekov (1998) +2 other references |
| Okrugin et al. (2023) +1 other reference |
| Kiseleva et al. (2014) |
| Pavel.M. Kartashov (n.d.) |
| Stepanov et al. (2023) |
| Stepanov et al. (2023) | |
| Sharygin et al. (2022) |
| Pavel.M. Kartashov (n.d.) |
| Stepanov et al. (2019) |
| Stepanov et al. (2019) | |
| Stepanov et al. (2019) | |
| Stepanov et al. (2019) | |
| Garuti et al. (2002) |
| |
| Johan (2006) |
| Pekov (1998) | |
| Pavel.M. Kartashov (n.d.) | |
| Garuti et al. (2002) +2 other references |
| Oidup et al. (2012) |
| Seltmann et al. (2010) |
Serbia | |
| Krstic et al. (1997) |
Sierra Leone | |
| Bowles et al. (2018) |
Slovakia | |
| Radvanec et al. (2016) |
South Africa | |
| Melcher et al. (2007) |
| Oberthür et al. (2021) |
| Cairncross et al. (1995) | |
Tanzania | |
| Evans et al. (2012) |
Turkey | |
| Kozlu et al. (2010) |
| Uysal et al. (2009) |
UK | |
| Anthony et al. (1990) |
| Anthony et al. (1990) | |
| Prichard et al. (1988) | |
| Prichard et al. (1988) +1 other reference | |
USA | |
| Canadian Mineralogist: 22: 543-552. |
| Snetsinger (1973) | |
| USGS Open-File Report 01-269 +1 other reference | |
| Anthony et al. (1990) | |
| USGS Open-File Report 01-269 | |
| - (2008) |
| - (2008) | |
| - (2008) | |
| - (2008) | |
| - (2008) | |
| - (2008) | |
| Silliman et al. (1873c) +1 other reference |
| - (2005) |
| Hittell (1868) +1 other reference |
| Richthofen (1865) +1 other reference |
| Horton (1912) +1 other reference |
| Min Mag (1997) |
| Van Nostrand Reinholt Press: 41. +2 other references |
| Van Nostrand Reinholt Press: 45. +2 other references |
| Genth (1852) +2 other references |
| Van Nostrand Reinholt Press: 43. +2 other references |
| Mining and Scientific Press (1901) +1 other reference |
| - (2005) +2 other references |
| Horton (1912) +1 other reference |
| - (2005) +1 other reference | |
| Petrov (n.d.) +1 other reference |
| Van Nostrand Reinholt Press: 45. +2 other references |
| - (2005) |
| Genth (1852) +2 other references |
| Van Nostrand Reinholt Press: 45. +2 other references |
| NBMG ofr 88-1 |
| - (2005) |
| - (2005) | |
| Bird et al. (1980) |
| - (2005) |
| - (2005) |
| - (2005) | |
| Lyon et al. (1997) |
| ... |
| Smith et al. (2008) +1 other reference |
| Derkey et al. (1990, November) +1 other reference |
Zimbabwe | |
| Oberthür et al. (2013) |
Quick NavTopAbout OsmiumUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentSynonymsOther LanguagesVarietiesCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List


 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Adamsfield, Derwent Valley municipality, Tasmania, Australia