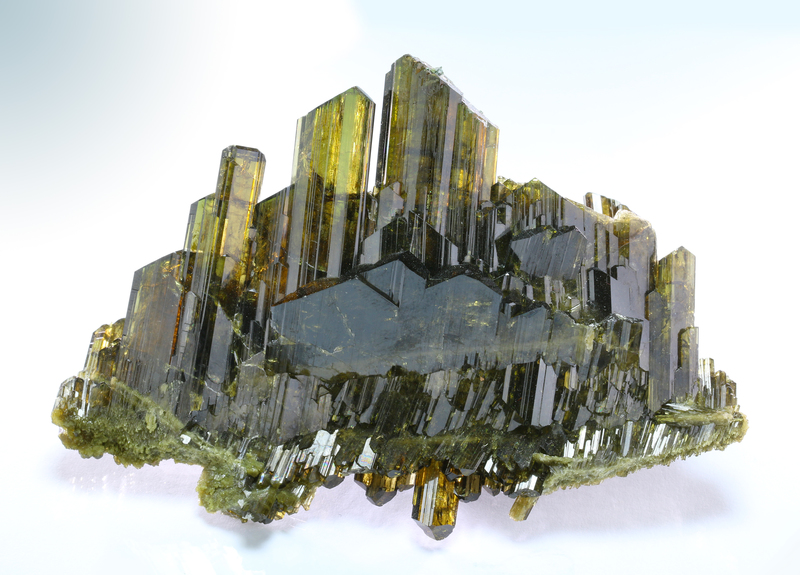
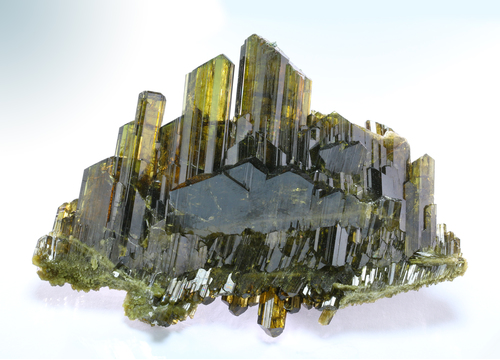
Epidote
A valid IMA mineral species - grandfathered
This page kindly sponsored in memory of Laszlo Z. Valachi
About Epidote
Formula:
(CaCa)(AlAlFe3+)O[Si2O7][SiO4](OH)
Colour:
Yellowish-green, green, brownish-green, black
Lustre:
Vitreous
Hardness:
6
Specific Gravity:
3.38 - 3.49
Crystal System:
Monoclinic
Member of:
Name:
Named in 1801 by Rene Just Haüy from the Greek επιδοσιζ ("epidosis"), meaning "increase", in allusion to the crystal characteristic of one longer side at the base of the prism.
Member of the Clinozoisite Group of the Epidote Supergroup.
Clinozoisite-Epidote Series. The Al2Fe3+ analogue of Clinozoisite.
Piemontite-Epidote Series. The Al2Fe3+ analogue of Piemontite.
 Visit gemdat.org for gemological information about Epidote.
Visit gemdat.org for gemological information about Epidote.
Clinozoisite-Epidote Series. The Al2Fe3+ analogue of Clinozoisite.
Piemontite-Epidote Series. The Al2Fe3+ analogue of Piemontite.
 Visit gemdat.org for gemological information about Epidote.
Visit gemdat.org for gemological information about Epidote.Unique Identifiers
Mindat ID:
1389
Long-form identifier:
mindat:1:1:1389:1
GUID
(UUID V4):
(UUID V4):
ad7fdfab-07df-483c-a51d-c515b0234bcd
IMA Classification of Epidote
Approved, 'Grandfathered' (first described prior to 1959)
IMA Formula:
Ca2(Al2Fe3+)[Si2O7][SiO4]O(OH)
Classification of Epidote
9.BG.05a
9 : SILICATES (Germanates)
B : Sorosilicates
G : Sorosilicates with mixed SiO4 and Si2O7 groups; cations in octahedral [6] and greater coordination
9 : SILICATES (Germanates)
B : Sorosilicates
G : Sorosilicates with mixed SiO4 and Si2O7 groups; cations in octahedral [6] and greater coordination
58.2.1a.7
58 : SOROSILICATES Insular, Mixed, Single, and Larger Tetrahedral Groups
2 : Insular, Mixed, Single, and Larger Tetrahedral Groups with cations in [6] and higher coordination; single and double groups (n = 1, 2)
58 : SOROSILICATES Insular, Mixed, Single, and Larger Tetrahedral Groups
2 : Insular, Mixed, Single, and Larger Tetrahedral Groups with cations in [6] and higher coordination; single and double groups (n = 1, 2)
16.21.2
16 : Silicates Containing Aluminum and other Metals
21 : Aluminosilicates of Fe and Ca
16 : Silicates Containing Aluminum and other Metals
21 : Aluminosilicates of Fe and Ca
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Ep | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Ep | Kretz (1983) | Kretz, R. (1983) Symbols of rock-forming minerals. American Mineralogist, 68, 277–279. |
| Ep | Siivolam & Schmid (2007) | Siivolam, J. and Schmid, R. (2007) Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: List of mineral abbreviations. Web-version 01.02.07. IUGS Commission on the Systematics in Petrology. download |
| Ep | Whitney & Evans (2010) | Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 doi:10.2138/am.2010.3371 |
| Ep | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
| Ep | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Pronunciation of Epidote
Pronunciation:
| Play | Recorded by | Country |
|---|---|---|
| Jolyon Ralph | United Kingdom |
Physical Properties of Epidote
Vitreous
Transparency:
Transparent, Translucent, Opaque
Colour:
Yellowish-green, green, brownish-green, black
Streak:
Colourless
Hardness:
6 on Mohs scale
Tenacity:
Brittle
Cleavage:
Perfect
Perfect on {001}, imperfect on {100}
Perfect on {001}, imperfect on {100}
Fracture:
Irregular/Uneven
Density:
3.38 - 3.49 g/cm3 (Measured) 3.43(3) g/cm3 (Calculated)
Optical Data of Epidote
Type:
Biaxial (-)
RI values:
nα = 1.715 - 1.751 nβ = 1.725 - 1.784 nγ = 1.734 - 1.797
2V:
Measured: 90° to 116°, Calculated: 62° to 84°
Max Birefringence:
δ = 0.019 - 0.046

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
High
Dispersion:
strong r > v
Pleochroism:
Strong
Comments:
X= colourless, pale yellow, pale green
Y= greenish yellow
Z= yellowish green
Y= greenish yellow
Z= yellowish green
Chemistry of Epidote
Mindat Formula:
(CaCa)(AlAlFe3+)O[Si2O7][SiO4](OH)
Common Impurities:
Al,Mg,Mn
Crystallography of Epidote
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P21/m
Cell Parameters:
a = 8.8877(14) Å, b = 5.6275(8) Å, c = 10.1517(12) Å
β = 115.383(14)°
β = 115.383(14)°
Ratio:
a:b:c = 1.579 : 1 : 1.804
Unit Cell V:
458.73 ų (Calculated from Unit Cell)
Z:
2
Morphology:
Crystals prismatic to 35 cm, also stubby, rarely tabular or pseudo-octahedral. Fibrous, coarse to finely granular, massive. Prismatic crystals may show a pseudo-hexagonal cross-section.
Twinning:
On {100}, contact, lamellar, common.
Crystallographic forms of Epidote
Crystal Atlas:
Image Loading
Click on an icon to view
3d models and HTML5 code kindly provided by
www.smorf.nl.
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0000041 | Epidote | Ito T (1947) The structure of epidote (HCa2(Al,Fe)Al2Si3O13) American Mineralogist 32 309-321 |  | 1947 | 0 | 293 | |
| 0000226 | Epidote | Dollase W A (1971) Refinement of the crystal structures of epidote, allanite and hancockite American Mineralogist 56 447-464 |  | 1971 | 0 | 293 | |
| 0000229 | Epidote | Dollase W A (1971) Refinement of the crystal structures of epidote, allanite and hancockite American Mineralogist 56 447-464 |  | 1971 | 0 | 293 | |
| 0000308 | Epidote | Gabe E J, Portheine J C, Whitlow S H (1973) A reinvestigation of the epidote structure: Confirmation of the iron location sample HEP American Mineralogist 58 218-223 |  | 1973 | 0 | 293 | |
| 0000309 | Epidote | Gabe E J, Portheine J C, Whitlow S H (1973) A reinvestigation of the epidote structure: Confirmation of the iron location sample LEP American Mineralogist 58 218-223 |  | 1973 | 0 | 293 | |
| 0002248 | Epidote | Giuli G, Bonazzi P, Menchetti S (1999) Al-Fe disorder in synthetic epidotes: A single-crystal X-ray diffraction study American Mineralogist 84 933-936 |  | 1999 | 0 | 293 | |
| 0016949 | Epidote | Nagashima M, Akasada M (2010) X-ray Rietveld and 57Fe Mossbauer studies of epidote and piemontite on the join Ca2Al2FeSi3O12(OH) - Ca2Al2MnSi3O12(OH) formed by hydrothermal synthesis American Mineralogist 95 1237-1246 | 2010 | synthetic | 0 | 293 | |
| 0016950 | Epidote | Nagashima M, Akasada M (2010) X-ray Rietveld and 57Fe Mossbauer studies of epidote and piemontite on the join Ca2Al2FeSi3O12(OH) - Ca2Al2MnSi3O12(OH) formed by hydrothermal synthesis American Mineralogist 95 1237-1246 | 2010 | synthetic | 0 | 293 | |
| 0016951 | Epidote | Nagashima M, Akasada M (2010) X-ray Rietveld and 57Fe Mossbauer studies of epidote and piemontite on the join Ca2Al2FeSi3O12(OH) - Ca2Al2MnSi3O12(OH) formed by hydrothermal synthesis American Mineralogist 95 1237-1246 | 2010 | synthetic | 0 | 293 | |
| 0016952 | Epidote | Nagashima M, Akasada M (2010) X-ray Rietveld and 57Fe Mossbauer studies of epidote and piemontite on the join Ca2Al2FeSi3O12(OH) - Ca2Al2MnSi3O12(OH) formed by hydrothermal synthesis American Mineralogist 95 1237-1246 | 2010 | synthetic | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Image Loading
Radiation - Copper Kα
Data courtesy of RRUFF project at University of Arizona, used with permission.
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 2.900 Å | (100) |
| 2.679 Å | (100) |
| 2.688 Å | (70) |
| 4.02 Å | (50) |
| 2.599 Å | (50) |
| 2.460 Å | (50) |
| 3.40 Å | (40) |
Comments:
Bourg d'Oisans, France.
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 3b: Earth’s earliest hydrosphere | >4.45 |
| 16 : Low-𝑇 aqueous alteration of Hadean subaerial lithologies (see also #23) | |
| Near-surface Processes | |
| 22 : Hydration and low-𝑇 subsurface aqueous alteration (see also #23) | |
| High-𝑇 alteration and/or metamorphism | |
| 31 : Thermally altered carbonate, phosphate, and iron formations | |
| Stage 5: Initiation of plate tectonics | <3.5-2.5 |
| 39 : High-𝑃 metamorphism (blueschist, eclogite, ultrahigh 𝑃 facies) | |
| 40 : Regional metamorphism (greenschist, amphibolite, granulite facies) | |
| 43 : Shear-induced minerals (including mylonite/slickensides) |
Geological Setting:
Regional and contact metamorphic rocks. Saussuritisation (alteration of plagioclase).
Type Occurrence of Epidote
Place of Conservation of Type Material:
Muséum Nationale d’Histoire Naturelle, Paris, France, numbers H3408 and H3445 (cotype).
Synonyms of Epidote
Other Language Names for Epidote
Croatian:Epidot
Dutch:Epidoot
Finnish:Epidootti
French:Épidote
Pistachite
Pistachite
German:Epidot
Acanthikon
Acanticonit
Acantikonit
Achmatit
Aescherit
Allochit
Arendalit
Arendit
Eisenepidot
Epidotit
Escherit
Pistacit
Pistazit
Posstrevorit
Puschkinit
Pushkinit
Selphinit
Thallit
Acanthikon
Acanticonit
Acantikonit
Achmatit
Aescherit
Allochit
Arendalit
Arendit
Eisenepidot
Epidotit
Escherit
Pistacit
Pistazit
Posstrevorit
Puschkinit
Pushkinit
Selphinit
Thallit
Hebrew:אפידוט
Hungarian:Epidot
Italian:Epidoto
Japanese:緑簾石
Lithuanian:Epidotas
Polish:Epidot
Portuguese:Epídoto
Romanian:Epidot
Russian:Эпидот
Simplified Chinese:绿帘石
Slovak:Epidot
Spanish:Epidota
Acanticonita
Achmatita
Aescherita
Allochita
Arendalita
Arendita
Epidotita
Escherita
Pistacita
Posstrevorita
Puschkinita
Pushkinita
Selphinita
Thallita
Acanticonita
Achmatita
Aescherita
Allochita
Arendalita
Arendita
Epidotita
Escherita
Pistacita
Posstrevorita
Puschkinita
Pushkinita
Selphinita
Thallita
Swedish:Epidot
Traditional Chinese:綠簾石
Ukrainian:Епідот
Varieties of Epidote
| Allanite-Epidote | Epidote enriched with REE and Y transitive to allanite-(Ce) or -(Y). Its REE+Y content is below 0.5 apfu. Sometimes such epidotes are selectively enriched in Eu (as at the Tsakhirin Khuduk deposit in Mongolian Altai). |
| Beryllium-bearing Epidote | A beryllium-bearing variety of epidote. |
| Bucklandite (of Hermann) | Morphological variety of dipyramidal Epidote. It is usually dark-colored, REE-bearing, transitive to Allanite-(Ce). The first description of the variety was made by Hermann in 1833 from Akhmatovskaya Kop' (Southern Ural). See also Bucklandite (of Lévy) |
| Rosstrevorite | A fibrous stellate variety of epidote, from Rosstrevor, Co. Down, Ireland (Greg and Lettsom, 1858). |
| Tawmawite | A Cr-bearing epidote. Originally reported from Tawmaw (Tawhmaw; Taw Maw), Myitkyina-Mogaung District, Kachin State, Myanmar (Burma). |
| Withamite | A Mn-rich variety of epidote. Originally reported from Glen Coe, Strathclyde (Argyllshire), Scotland, UK. May be confused with pinkish varieties of clinozoisite and zoisite ("thulite"). |
| Yttroepidote | Yttrium-bearing epidote with a Y+REE content below 0.5 apfu. Transitive to allanite-(Y). First described from Slyudorudnik, Southern Urals, in 1959. |
Relationship of Epidote to other Species
Member of:
Other Members of this group:
| Clinozoisite | (CaCa)(AlAlAl)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Epidote-(Sr) | (CaSr)(AlAlFe3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Hancockite | (CaPb)(AlAlFe3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Heflikite | (CaCa)(AlAlSc)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Mukhinite | (CaCa)(AlAlV3+)O[Si2O7][SiO4](OH) | Mon. |
| Niigataite | (CaSr)(AlAlAl)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Piemontite | (CaCa)(AlAlMn3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Piemontite-(Pb) | (CaPb)(AlAlMn3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Piemontite-(Sr) | (CaSr)(AlAlMn3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Tweddillite | (CaSr)(Mn3+AlMn3+)O[Si2O7][SiO4](OH) | Mon. 2/m : P21/m |
| Unnamed (Fe3+ analogue of Piemontite-(Pb)) | (CaPb)(Fe3+AlMn3+)O[Si2O7][SiO4](OH) | |
| Unnamed (Fe3+-analogue of Piemontite) | (CaCa)(Fe3+AlMn3+)O[Si2O7][SiO4](OH) | |
| Unnamed (Fe3+-analogue of Piemontite-(Sr)) | (CaSr)(Fe3+AlMn3+)O[Si2O7][SiO4](OH) | |
| Unnamed (Ga-analogue of Epidote) | (CaCa)(AlAlGa3+)O[Si2O7][SiO4](OH) |
Forms a series with:
Common Associates
Associated Minerals Based on Photo Data:
| 2,258 photos of Epidote associated with Quartz | SiO2 |
| 1,264 photos of Epidote associated with Prehnite | Ca2Al2Si3O10(OH)2 |
| 616 photos of Epidote associated with Calcite | CaCO3 |
| 461 photos of Epidote associated with Albite | Na(AlSi3O8) |
| 348 photos of Epidote associated with Andradite | Ca3Fe3+2(SiO4)3 |
| 316 photos of Epidote associated with Byssolite | AX2Z5((Si,Al,Ti)8O22)(OH,F,Cl,O)2 |
| 267 photos of Epidote associated with Diopside | CaMgSi2O6 |
| 256 photos of Epidote associated with Magnetite | Fe2+Fe3+2O4 |
| 245 photos of Epidote associated with Copper | Cu |
| 239 photos of Epidote associated with Titanite | CaTi(SiO4)O |
Related Minerals - Strunz-mindat Grouping
| 9.BG. | Shuiskite-(Cr) | Ca2Cr3+Cr3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG. | Alnaperbøeite-(Ce) | Ca(Ce2.5Na0.5)(AlAl2Al)[Si2O7][SiO4]3O(OH)2 |
| 9.BG. | Magnesiovesuvianite | Ca19MgAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 |
| 9.BG. | Alumovesuvianite | Ca19AlAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG. | Zoisite-(Pb) | (CaPb)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG. | Vielleaureite-(Ce) | (Mn2+Ce)(MgAlMn2+)F[Si2O7][SiO4](OH) |
| 9.BG. | Heflikite | (CaCa)(AlAlSc)O[Si2O7][SiO4](OH) |
| 9.BG. | Zilbermintsite-(La) | (CaLa5)(Fe3+Al3Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.05b | Allanite-(Ce) | (CaCe)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(La) | (CaLa)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Y) | (CaY)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Clinozoisite | (CaCa)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Dissakisite-(Ce) | (CaCe)(AlAlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Dollaseite-(Ce) | (CaCe)(MgAlMg)F[Si2O7][SiO4](OH) |
| 9.BG.05a | Hancockite | (CaPb)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Khristovite-(Ce) | (CaCe)(MgAlMn2+)F[Si2O7][SiO4](OH) |
| 9.BG.05a | Mukhinite | (CaCa)(AlAlV3+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Piemontite | (CaCa)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Piemontite-(Sr) | (CaSr)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Manganiandrosite-(La) | (Mn2+La)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Tweddillite | (CaSr)(Mn3+AlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriallanite-(Ce) | (CaCe)(Fe3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Niigataite | (CaSr)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Manganiandrosite-(Ce) | (Mn2+Ce)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Dissakisite-(La) | (CaLa)(AlAlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Vanadoandrosite-(Ce) | (Mn2+Ce)(V3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Uedaite-(Ce) | (Mn2+Ce)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Epidote-(Sr) | (CaSr)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Nd) | (CaNd)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Unnamed (Mg-analogue of Ferriallanite-(Ce)) | (CaCe)(Fe3+AlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Unnamed (Mn3+-analogue of Ferriakasakaite-(Ce)) | (CaCe)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriallanite-(La) | (CaLa)(Fe3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Åskagenite-(Nd) | (Mn2+Nd)(AlAlFe3+)O[Si2O7][SiO4]O |
| 9.BG.05 | Piemontite-(Pb) | (CaPb)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Vanadoallanite-(La) | (CaLa)(V3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriakasakaite-(La) | (CaLa)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Ferriandrosite-(La) | (Mn2+La)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Androsite-(Ce) | (Mn2+Ce)(AlAlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Ferriandrosite-(Ce) | (Mn2+Ce)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a v | Unnamed (Ga-analogue of Epidote) | (CaCa)(AlAlGa3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | UM1989-32-SiO:AlCaFeHREE | (Ca0.5◻0.5REE)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Manganiakasakaite-(La) | (CaLa)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriakasakaite-(Ce) | (CaCe)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Sm) | (CaSm)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.10 | Zoisite | (CaCa)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.15 | Macfallite | Ca2Mn3+3(SiO4)(Si2O7)(OH)3 |
| 9.BG.15 | Sursassite | Mn2+2Al3(SiO4)(Si2O7)(OH)3 |
| 9.BG.20 | Julgoldite-(Fe2+) | Ca2Fe2+Fe3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Okhotskite | Ca2Mn2+Mn3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Pumpellyite-(Fe2+) | Ca2Fe2+Al2(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Pumpellyite-(Fe3+) | Ca2Fe3+Al2(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Pumpellyite-(Mg) | Ca2MgAl2(Si2O7)(SiO4)(OH)2 · H2O |
| 9.BG.20 | Pumpellyite-(Mn2+) | Ca2Mn2+Al2(Si2O7)(SiO4)(OH)2 · H2O |
| 9.BG.20 | Shuiskite-(Mg) | Ca2MgCr3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Julgoldite-(Fe3+) | Ca2Fe3+Fe3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG.20 | Pumpellyite-(Al) | Ca2Al3(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Poppiite | Ca2V3+V3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG.20 | Julgoldite-(Mg) | Ca2MgFe3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.25 | Ganomalite | Pb9Ca5Mn(Si2O7)4(SiO4)O |
| 9.BG.25 | Wayneburnhamite | Pb9Ca6(Si2O7)3(SiO4)3 |
| 9.BG.30 | Rustumite | Ca10(Si2O7)2(SiO4)(OH)2Cl2 |
| 9.BG.35 | Vesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG.35 | Wiluite | Ca19MgAl4(Al,Mg)8(B,◻)4◻[Si2O7]4[(SiO4)10]O(O,OH)9 |
| 9.BG.35 | Manganvesuvianite | Ca19Mn3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG.35 | Fluorvesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(F,OH)9 |
| 9.BG.35 | Cyprine | Ca19Cu2+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 |
| 9.BG.35 | Hongheite | Ca19Fe2+Al4(Fe3+,Mg)8(◻4)B[Si2O7]4[(SiO4)10]O(OH,O)9 |
| 9.BG.35 | Milanriederite | (Ca18[REE])Fe3+Al4(Mg4Al4)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 |
| 9.BG.35 | Manaevite-(Ce) | (Ca13Ce4[H2O]2)Mg(Al3Mg)(Mg3Ti3Fe3+2)(◻4)◻[Si2O7]4[(SiO4)8(H4O4)2]O(OH)9 |
| 9.BG.40 | Vyuntspakhkite-(Y) | (Y,Yb)4Al2.5-1.5(Si,Al)1.5-2.5(SiO4)4O(OH)7 |
| 9.BG.45 | Dellaite | Ca6Si3O11(OH)2 |
| 9.BG.50 | Gatelite-(Ce) | CaCe3(AlAl2Mg)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Perbøeite-(Ce) | CaCe3(AlAl2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Ferriperbøeite-(Ce) | CaCe3(Fe3+Al2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Ferriperbøeite-(La) | CaLa3(Fe3+Al2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Perbøeite-(La) | CaLa3(AlAl2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.55 | Västmanlandite-(Ce) | CaCe3(MgAl2Mg)[Si2O7][SiO4]3F(OH)2 |
| 9.BG.60 | Radekškodaite-(La) | (CaLa5)(Al4Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.60 | Radekškodaite-(Ce) | (CaCe5)(Al4Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.60 | Radekškodaite Group | (CaM5)([Fe3+Al3]Fe2+)[Si2O7][SiO4]5O(OH)3 |
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Epidote in petrology
An essential component of rock names highlighted in red, an accessory component in rock names highlighted in green.
Internet Links for Epidote
mindat.org URL:
https://www.mindat.org/min-1389.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Epidote
Reference List:
Dollase, W. A. (1971) Refinement of the crystal structures of epidote, allanite, and hancockite. American Mineralogist, 56 (3-4) 447-464
Janeczek, Janusz, Sachanbinski, Michael (1992) Babingtonite, Y-Al-rich titanite, and zoned epidote from the Strzegom pegmatites, Poland. European Journal of Mineralogy, 4 (2) 307-320 doi:10.1127/ejm/4/2/0307
Armbruster, Thomas, Bonazzi, Paola, Akasaka, Masahide, Bermanec, Vladimir, Chopin, Christian, Gieré, Reto, Heuss-Assbichler, Soraya, Liebscher, Axel, Menchetti, Silvio, Pan, Yuanming, Pasero, Marco (2006) Recommended nomenclature of epidote-group minerals. European Journal of Mineralogy, 18 (5) 551-567 doi:10.1127/0935-1221/2006/0018-0551
Significant localities for Epidote
Showing 22 significant localities out of 10,282 recorded on mindat.org.
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Austria | |
| Niedermayr et al. (1995) |
| Mason (1976) +1 other reference |
Belgium | |
| Stöber (1895) +2 other references |
Ecuador | |
| Alejandro Felix Gutierrez |
France (TL) | |
| Haüy (1801a) +1 other reference |
| Piccoli (2002) +1 other reference |
| Serge Lavarde Collection |
| Serge Lavarde Collection | |
Guatemala | |
| USGS Bulletin 1034 |
Italy | |
| Borson (1811) +15 other references |
| Alpinisti M. (1981) +1 other reference |
Mexico | |
| Peninsular Range Collection - Curtis ... |
North Macedonia | |
| Bermanec et al. (2001) +1 other reference |
Pakistan | |
| Weerth (1991) |
Portugal | |
| |
South Africa | |
| PMPB Meulenbeld collection Photo ID: ... |
UK | |
| [var: Withamite] Hutton +2 other references |
USA | |
| Mason (1976) +1 other reference |
| Jake Harper: Field work |
| Betts (1999) |
| Davis (1901) +2 other references |
| Harvard Mineralogical Museum No. 119199 +1 other reference |
| Benham et al. (1985) |
Quick NavTopAbout EpidoteUnique IdentifiersIMA Classification Classification Mineral SymbolsPronunciation Physical Properties Optical Data Chemistry Crystallography Crystallographic forms Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesVarietiesRelationshipsCommon AssociatesStrunz-MindatOther InformationEpidote in petrologyInternet Links References Significant localities Locality List




 symbol to view information about a locality.
The
symbol to view information about a locality.
The 





Alchuri alpine-type clefts, Alchuri, Shigar Valley, Shigar District, Gilgit-Baltistan, Pakistan