Cyprine
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Cyprine
Formula:
Ca19Cu2+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9
The formula is a partial simplification of the full structural formula. The large (VII-IX)-coordinated (X4)2(X3)8(X2)8(X1) sites are here combined (e.g. Ca19) and are typically filled with Ca, although other large cations such as the REE may be present. The square-pyramidal Y1 site can host a variety of M2+ and M3+ ions and is the basis for the distinction of several species. The VI-coordinated Y2 site typically is filled with Al, whereas the also VI-coordinated Y3 site may contain Al, Mg, and other cations of similar charge and size. The tetrahedral T1 site is typically vacant but may contain B (less commonly Al); the trigonal T2 site is also typically vacant but may also contain B. Some of the (SiO4) may be replaced by (H4O4), akin to the Si4+ ↔︎ 4H+ hydrogarnet substitution. Among the oxygen that are not part of the silica tetrahedra, there are eight "O11" that typically occur as OH, two "O10" that are typically O & OH or OH & OH (the latter arrangement notably when Y1 is an M2+ cation). There may also be up to three "O12" that in most vesuvianite-group minerals are absent (and are not included here), but may be present particularly when T1 is occupied.
Colour:
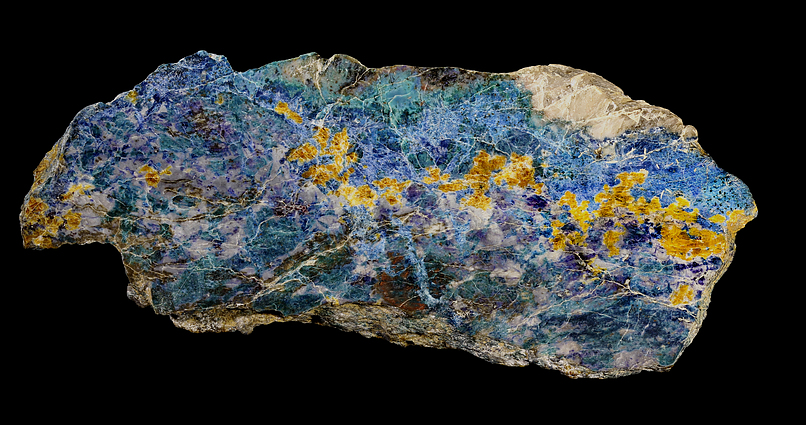
Blue, bluish green, green, dark red with a lilac hue (type material),
Lustre:
Vitreous, Sub-Vitreous
Hardness:
6½
Specific Gravity:
3.40
Crystal System:
Tetragonal
Member of:
Name:
The historical name cyprine (derived from Latin cuprum, copper) was given by Jöns Jakob Berzelius in 1821 for a Cu-bearing vesuvianite. By 1892, Edward S. Dana listed cyprine as a blue vesuvianite containing "trace amounts" of copper. In 1986, Sharon Fitzgerald, A. L. Rheingold, and Peter B. Leavens, studied the crystal structure of cyprines and found that Cu2+ occurred in only one site and that in some instances, Cu2+ was the dominant cation in that site. In 2017, T. L. Panikorovskii, V. V. Shilovskikh, E. Yu. Avdontseva A. A, Zolotarev, Ivan V. Pekov, S. N. Britvin, and S. V. Krivovichev reported on a highly manganian vesuvianite that had insufficient Mn3+ to qualify as a new species, but that there was dominant Cu2+ in the site as had been previously identified by Fitzgerald, et. al. (1986). On the basis of the ordered Cu2+, cyprine was re-introduced as a copper-bearing species in the vesuvianite group.
The name 'cyprine' was previously given to any blue variety of vesuvianite on the (not always correct) assumption that it contained abundant copper.
Some blue 'cyprine' specimens may in fact contain sufficient copper, but many specimens do not contain enough for a Cu-dominant occupancy (Cu >0.5 apfu) of the Y1 cation site in the crystal structure. Consequently some samples labelled as 'cyprine' have been misidentified, although some specimens from Norway and Franklin, New Jersey, USA contain sufficient Cu2+ in the Y1 site to be called cyprine by the current definition.
Some blue 'cyprine' specimens may in fact contain sufficient copper, but many specimens do not contain enough for a Cu-dominant occupancy (Cu >0.5 apfu) of the Y1 cation site in the crystal structure. Consequently some samples labelled as 'cyprine' have been misidentified, although some specimens from Norway and Franklin, New Jersey, USA contain sufficient Cu2+ in the Y1 site to be called cyprine by the current definition.
Unique Identifiers
Mindat ID:
46798
Long-form identifier:
mindat:1:1:46798:2
GUID
(UUID V4):
(UUID V4):
12d83ce2-83ca-4286-87d4-3bf3e8a07885
IMA Classification of Cyprine
Approved
IMA Formula:
Ca19Cu2+(Al,Mg)12Si18O69(OH)9
Approval year:
2015
First published:
2017
Type description reference:
Panikorovskii, Taras L., Shilovskikh, Vladimir V., Avdontseva, Evgenia Yu., Zolotarev, Andrey A., Pekov, Igor V., Britvin, Sergey N., Hålenius, Ulf, Krivovichev, Sergey V. (2017) Cyprine, Ca19Cu2+(Al,Mg,Mn)12Si18O69(OH)9, a new vesuvianite-group mineral from the Wessels mine, South Africa. European Journal of Mineralogy, 29 (2) 295-306 doi:10.1127/ejm/2017/0029-2592
Classification of Cyprine
9.BG.35
9 : SILICATES (Germanates)
B : Sorosilicates
G : Sorosilicates with mixed SiO4 and Si2O7 groups; cations in octahedral [6] and greater coordination
9 : SILICATES (Germanates)
B : Sorosilicates
G : Sorosilicates with mixed SiO4 and Si2O7 groups; cations in octahedral [6] and greater coordination
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Cyp | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Pronunciation of Cyprine
Pronunciation:
| Play | Recorded by | Country |
|---|---|---|
| Jolyon Ralph | United Kingdom |
Physical Properties of Cyprine
Vitreous, Sub-Vitreous
Transparency:
Transparent
Colour:
Blue, bluish green, green, dark red with a lilac hue (type material),
Comment:
Color on single crystals
Streak:
Usually white to pale tints of the body color
Hardness:
6½ on Mohs scale
Tenacity:
Brittle
Cleavage:
None Observed
Fracture:
Irregular/Uneven, Splintery, Hackly, Sub-Conchoidal
Density:
3.40 g/cm3 (Measured) 3.41 g/cm3 (Calculated)
Optical Data of Cyprine
Type:
Uniaxial (-)
RI values:
nω = 1.744(2) nε = 1.732(2)
Birefringence:
May show anomalous birefringence; the birefringence may also be variably obscured by the body color of the mineral in strongly-colored examples.
Max Birefringence:
δ = 0.012

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
High
Pleochroism:
Strong
Comments:
Ranging from O = dark purple, E = pale red to O = dark reddish brown, E = pale yellowish brown; O>>E.
Chemistry of Cyprine
Mindat Formula:
Ca19Cu2+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9
The formula is a partial simplification of the full structural formula. The large (VII-IX)-coordinated (X4)2(X3)8(X2)8(X1) sites are here combined (e.g. Ca19) and are typically filled with Ca, although other large cations such as the REE may be present. The square-pyramidal Y1 site can host a variety of M2+ and M3+ ions and is the basis for the distinction of several species. The VI-coordinated Y2 site typically is filled with Al, whereas the also VI-coordinated Y3 site may contain Al, Mg, and other cations of similar charge and size. The tetrahedral T1 site is typically vacant but may contain B (less commonly Al); the trigonal T2 site is also typically vacant but may also contain B. Some of the (SiO4) may be replaced by (H4O4), akin to the Si4+ ↔︎ 4H+ hydrogarnet substitution. Among the oxygen that are not part of the silica tetrahedra, there are eight "O11" that typically occur as OH, two "O10" that are typically O & OH or OH & OH (the latter arrangement notably when Y1 is an M2+ cation). There may also be up to three "O12" that in most vesuvianite-group minerals are absent (and are not included here), but may be present particularly when T1 is occupied.
The formula is a partial simplification of the full structural formula. The large (VII-IX)-coordinated (X4)2(X3)8(X2)8(X1) sites are here combined (e.g. Ca19) and are typically filled with Ca, although other large cations such as the REE may be present. The square-pyramidal Y1 site can host a variety of M2+ and M3+ ions and is the basis for the distinction of several species. The VI-coordinated Y2 site typically is filled with Al, whereas the also VI-coordinated Y3 site may contain Al, Mg, and other cations of similar charge and size. The tetrahedral T1 site is typically vacant but may contain B (less commonly Al); the trigonal T2 site is also typically vacant but may also contain B. Some of the (SiO4) may be replaced by (H4O4), akin to the Si4+ ↔︎ 4H+ hydrogarnet substitution. Among the oxygen that are not part of the silica tetrahedra, there are eight "O11" that typically occur as OH, two "O10" that are typically O & OH or OH & OH (the latter arrangement notably when Y1 is an M2+ cation). There may also be up to three "O12" that in most vesuvianite-group minerals are absent (and are not included here), but may be present particularly when T1 is occupied.
Chemical Analysis
Oxide wt%:
| 1 | |
|---|---|
| SiO2 | 36.08 % |
| Al2O3 | 16.12 % |
| V2O3 | 0.01 % |
| Mn2O3* | 1.26 % |
| Fe2O3 | 0.81 % |
| MnO* | 4.78 % |
| NiO | 0.01 % |
| CuO | 2.34 % |
| MgO | 0.05 % |
| CaO | 34.42 % |
| SrO | 0.07 % |
| BaO | 0.01 % |
| Na2O | 0.08 % |
| K2O | 0.01 % |
| F | 0.34 % |
| H2O** | 2.52 % |
| -O=F | -0.15 % |
| Total: | 98.76 % |
Empirical formulas:
| Sample ID | Empirical Formula |
|---|---|
| 1 | (Ca18.56Mn2+0.33Na0.08Sr0.02K0.01)(Cu2+0.89Mn3+0.11)Al4.00(Al5.56Mn2+1.71Mn3+0.37Fe3+0.31Mg0.04)(◻4.83Si0.17)◻[Si2.00O7]4[Si0.998P0.002O4]10(O)([OH]8.45F0.55) |
Sample references:
| ID | Locality | Reference | Notes |
|---|---|---|---|
| 1 | N'Chwaning II Mine, N'Chwaning Mines, Joe Morolong Local Municipality, John Taolo Gaetsewe District Municipality, Northern Cape, South Africa | most Cu-rich region in zoned Mn-bearing cyprine/Cu-bearing manganvesuvianite. *Mn measured as Mn2+; relative Mn2O3 and MnO calculated by charge balance, assuming O(OH)9. **Likewise, H2O is calculated by stoichiometry, also assuming O(OH)9. |
Crystallography of Cyprine
Crystal System:
Tetragonal
Class (H-M):
4/m - Dipyramidal
Space Group:
P4/n
Setting:
P4/n
Cell Parameters:
a = 15.5652(5) Å, c = 11.7921(4) Å
Ratio:
a:c = 1 : 0.758
Unit Cell V:
2863.2 ų
Z:
2
Morphology:
Crystals up to 1 cm. Dominant forms: {100}, {110}, {001}, and {331}.
Twinning:
No twinning observed in type material.
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 5.89 Å | (12) |
| 3.482 Å | (10) |
| 3.007 Å | (12) |
| 2.950 Å | (47) |
| 2.752 Å | (100) |
| 2.594 Å | (76) |
| 2.459 Å | (35) |
| 1.622 Å | (28) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| High-𝑇 alteration and/or metamorphism | |
| 31 : Thermally altered carbonate, phosphate, and iron formations |
Type Occurrence of Cyprine
General Appearance of Type Material:
As chaotic aggregates (up to 5 cm across) in open cavities or embedded in coarse-grained colourless calcite.
Place of Conservation of Type Material:
Type material is deposited in the collections of the Mineralogical Museum of St. Petersburg State University, catalogue no. 1/19536.
Geological Setting of Type Material:
An assemblage of Mn- and Ca-bearing minerals, formed as a result of the hydrothermal activity.
Associated Minerals at Type Locality:
Reference:
Panikorovskii, T.L., Shilovskikh, V.V., Avdontseva, E.Yu., Zolotarev, A.A., Pekov, I.V., Britvin, S.N., Krivovichev, S.V. (2017): Cyprine, Ca19Cu2+(Al,Mg,Mn)12Si18O68(OH)10, a new vesuvianite-group mineral from the Wessels mine, South Africa. European Journal of Mineralogy, 29, 295-306.
Synonyms of Cyprine
Other Language Names for Cyprine
Spanish:Ciprina
Relationship of Cyprine to other Species
Member of:
Other Members of this group:
| Alumovesuvianite | Ca19AlAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 | Tet. 4/m : P4/n |
| Fluorvesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(F,OH)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
| Hongheite | Ca19Fe2+Al4(Fe3+,Mg)8(◻4)B[Si2O7]4[(SiO4)10]O(OH,O)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
| Magnesiovesuvianite | Ca19MgAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 | Tet. 4/m : P4/n |
| Manaevite-(Ce) | (Ca13Ce4[H2O]2)Mg(Al3Mg)(Mg3Ti3Fe3+2)(◻4)◻[Si2O7]4[(SiO4)8(H4O4)2]O(OH)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
| Manganvesuvianite | Ca19Mn3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 | Tet. 4/m : P4/n |
| Milanriederite | (Ca18[REE])Fe3+Al4(Mg4Al4)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
| Vesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
| Wiluite | Ca19MgAl4(Al,Mg)8(B,◻)4◻[Si2O7]4[(SiO4)10]O(O,OH)9 | Tet. 4/mmm (4/m 2/m 2/m) : P4/nnc |
Common Associates
Associated Minerals Based on Photo Data:
| 29 photos of Cyprine associated with Thulite | {Ca2}{Al,Mn3+3}(Si2O7)(SiO4)O(OH) |
| 11 photos of Cyprine associated with Fluorite | CaF2 |
| 10 photos of Cyprine associated with Andradite | Ca3Fe3+2(SiO4)3 |
| 9 photos of Cyprine associated with Calcite | CaCO3 |
| 9 photos of Cyprine associated with Quartz | SiO2 |
| 7 photos of Cyprine associated with Piemontite | (CaCa)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 5 photos of Cyprine associated with Grossular | Ca3Al2(SiO4)3 |
| 2 photos of Cyprine associated with Bustamite | CaMn2+(Si2O6) |
| 2 photos of Cyprine associated with Hyalophane | (K,Ba)[Al(Si,Al)Si2O8] |
| 1 photo of Cyprine associated with Hendricksite | KZn3(Si3Al)O10(OH)2 |
Related Minerals - Strunz-mindat Grouping
| 9.BG. | Shuiskite-(Cr) | Ca2Cr3+Cr3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG. | Alnaperbøeite-(Ce) | Ca(Ce2.5Na0.5)(AlAl2Al)[Si2O7][SiO4]3O(OH)2 |
| 9.BG. | Magnesiovesuvianite | Ca19MgAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 |
| 9.BG. | Alumovesuvianite | Ca19AlAl4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG. | Zoisite-(Pb) | (CaPb)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG. | Vielleaureite-(Ce) | (Mn2+Ce)(MgAlMn2+)F[Si2O7][SiO4](OH) |
| 9.BG. | Heflikite | (CaCa)(AlAlSc)O[Si2O7][SiO4](OH) |
| 9.BG. | Zilbermintsite-(La) | (CaLa5)(Fe3+Al3Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.05b | Allanite-(Ce) | (CaCe)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(La) | (CaLa)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Y) | (CaY)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Clinozoisite | (CaCa)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Dissakisite-(Ce) | (CaCe)(AlAlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Dollaseite-(Ce) | (CaCe)(MgAlMg)F[Si2O7][SiO4](OH) |
| 9.BG.05a | Epidote | (CaCa)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Hancockite | (CaPb)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Khristovite-(Ce) | (CaCe)(MgAlMn2+)F[Si2O7][SiO4](OH) |
| 9.BG.05a | Mukhinite | (CaCa)(AlAlV3+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Piemontite | (CaCa)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Piemontite-(Sr) | (CaSr)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Manganiandrosite-(La) | (Mn2+La)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Tweddillite | (CaSr)(Mn3+AlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriallanite-(Ce) | (CaCe)(Fe3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Niigataite | (CaSr)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Manganiandrosite-(Ce) | (Mn2+Ce)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Dissakisite-(La) | (CaLa)(AlAlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Vanadoandrosite-(Ce) | (Mn2+Ce)(V3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Uedaite-(Ce) | (Mn2+Ce)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a | Epidote-(Sr) | (CaSr)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Nd) | (CaNd)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Unnamed (Mg-analogue of Ferriallanite-(Ce)) | (CaCe)(Fe3+AlMg)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Unnamed (Mn3+-analogue of Ferriakasakaite-(Ce)) | (CaCe)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriallanite-(La) | (CaLa)(Fe3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Åskagenite-(Nd) | (Mn2+Nd)(AlAlFe3+)O[Si2O7][SiO4]O |
| 9.BG.05 | Piemontite-(Pb) | (CaPb)(AlAlMn3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Vanadoallanite-(La) | (CaLa)(V3+AlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriakasakaite-(La) | (CaLa)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Ferriandrosite-(La) | (Mn2+La)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Androsite-(Ce) | (Mn2+Ce)(AlAlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05 | Ferriandrosite-(Ce) | (Mn2+Ce)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05a v | Unnamed (Ga-analogue of Epidote) | (CaCa)(AlAlGa3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | UM1989-32-SiO:AlCaFeHREE | (Ca0.5◻0.5REE)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Manganiakasakaite-(La) | (CaLa)(Mn3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Ferriakasakaite-(Ce) | (CaCe)(Fe3+AlMn2+)O[Si2O7][SiO4](OH) |
| 9.BG.05b | Allanite-(Sm) | (CaSm)(AlAlFe2+)O[Si2O7][SiO4](OH) |
| 9.BG.10 | Zoisite | (CaCa)(AlAlAl)O[Si2O7][SiO4](OH) |
| 9.BG.15 | Macfallite | Ca2Mn3+3(SiO4)(Si2O7)(OH)3 |
| 9.BG.15 | Sursassite | Mn2+2Al3(SiO4)(Si2O7)(OH)3 |
| 9.BG.20 | Julgoldite-(Fe2+) | Ca2Fe2+Fe3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Okhotskite | Ca2Mn2+Mn3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Pumpellyite-(Fe2+) | Ca2Fe2+Al2(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Pumpellyite-(Fe3+) | Ca2Fe3+Al2(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Pumpellyite-(Mg) | Ca2MgAl2(Si2O7)(SiO4)(OH)2 · H2O |
| 9.BG.20 | Pumpellyite-(Mn2+) | Ca2Mn2+Al2(Si2O7)(SiO4)(OH)2 · H2O |
| 9.BG.20 | Shuiskite-(Mg) | Ca2MgCr3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.20 | Julgoldite-(Fe3+) | Ca2Fe3+Fe3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG.20 | Pumpellyite-(Al) | Ca2Al3(Si2O7)(SiO4)(OH,O)2 · H2O |
| 9.BG.20 | Poppiite | Ca2V3+V3+2[Si2O6OH][SiO4](OH)2O |
| 9.BG.20 | Julgoldite-(Mg) | Ca2MgFe3+2[Si2O6OH][SiO4](OH)2(OH) |
| 9.BG.25 | Ganomalite | Pb9Ca5Mn(Si2O7)4(SiO4)O |
| 9.BG.25 | Wayneburnhamite | Pb9Ca6(Si2O7)3(SiO4)3 |
| 9.BG.30 | Rustumite | Ca10(Si2O7)2(SiO4)(OH)2Cl2 |
| 9.BG.35 | Vesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG.35 | Wiluite | Ca19MgAl4(Al,Mg)8(B,◻)4◻[Si2O7]4[(SiO4)10]O(O,OH)9 |
| 9.BG.35 | Manganvesuvianite | Ca19Mn3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(OH)9 |
| 9.BG.35 | Fluorvesuvianite | Ca19Fe3+Al4(Al6Mg2)(◻4)◻[Si2O7]4[(SiO4)10]O(F,OH)9 |
| 9.BG.35 | Hongheite | Ca19Fe2+Al4(Fe3+,Mg)8(◻4)B[Si2O7]4[(SiO4)10]O(OH,O)9 |
| 9.BG.35 | Milanriederite | (Ca18[REE])Fe3+Al4(Mg4Al4)(◻4)◻[Si2O7]4[(SiO4)10](OH)(OH)9 |
| 9.BG.35 | Manaevite-(Ce) | (Ca13Ce4[H2O]2)Mg(Al3Mg)(Mg3Ti3Fe3+2)(◻4)◻[Si2O7]4[(SiO4)8(H4O4)2]O(OH)9 |
| 9.BG.40 | Vyuntspakhkite-(Y) | (Y,Yb)4Al2.5-1.5(Si,Al)1.5-2.5(SiO4)4O(OH)7 |
| 9.BG.45 | Dellaite | Ca6Si3O11(OH)2 |
| 9.BG.50 | Gatelite-(Ce) | CaCe3(AlAl2Mg)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Perbøeite-(Ce) | CaCe3(AlAl2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Ferriperbøeite-(Ce) | CaCe3(Fe3+Al2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Ferriperbøeite-(La) | CaLa3(Fe3+Al2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.50 | Perbøeite-(La) | CaLa3(AlAl2Fe2+)[Si2O7][SiO4]3O(OH)2 |
| 9.BG.55 | Västmanlandite-(Ce) | CaCe3(MgAl2Mg)[Si2O7][SiO4]3F(OH)2 |
| 9.BG.60 | Radekškodaite-(La) | (CaLa5)(Al4Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.60 | Radekškodaite-(Ce) | (CaCe5)(Al4Fe2+)[Si2O7][SiO4]5O(OH)3 |
| 9.BG.60 | Radekškodaite Group | (CaM5)([Fe3+Al3]Fe2+)[Si2O7][SiO4]5O(OH)3 |
Fluorescence of Cyprine
Not fluorescent in UV
Other Information
IR Spectrum:
Absorption bands in the IR spectrum are 443, 490, 574, 604, 671, 814, 905, 972, 1015, 3354, 3640cm-1.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Cyprine
mindat.org URL:
https://www.mindat.org/min-46798.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
References for Cyprine
Reference List:
Hålenius, U., Hatert, F., Pasero, M., Mills, S. J. (2015) New minerals and nomenclature modifications approved in 2015, CNMNC Newsletter No 27. Mineralogical Magazine, 79 (5) 1223-1230 doi:10.1180/minmag.2015.079.5.16
Panikorovskii, Taras L., Shilovskikh, Vladimir V., Avdontseva, Evgenia Yu., Zolotarev, Andrey A., Pekov, Igor V., Britvin, Sergey N., Hålenius, Ulf, Krivovichev, Sergey V. (2017) Cyprine, Ca19Cu2+(Al,Mg,Mn)12Si18O69(OH)9, a new vesuvianite-group mineral from the Wessels mine, South Africa. European Journal of Mineralogy, 29 (2) 295-306 doi:10.1127/ejm/2017/0029-2592
Localities for Cyprine
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Norway | |
| Kjerulf (1879) |
| Ohkawa et al. (1992) |
| Berzelius (1820) +1 other reference |
| Larsen (2016) |
| Jan Andersen photo & specimen |
South Africa | |
| Frank K. Mazdab collection +2 other references |
| Hålenius et al. (2015) +1 other reference |
Sweden | |
| Gatedal (n.d.) |
| Gatedal (n.d.) |
USA | |
| Palache (1935) +2 other references |
| Northrop et al. (1996) |
Quick NavTopAbout CyprineUnique IdentifiersIMA Classification Classification Mineral SymbolsPronunciation Physical Properties Optical Data Chemistry Chemical AnalysisCrystallography X-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesRelationshipsCommon AssociatesStrunz-MindatFluorescence Other InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 




Wessels Mine, Joe Morolong Local Municipality, John Taolo Gaetsewe District Municipality, Northern Cape, South Africa