Exact matching result shown. To search for other matches click here
Redirected from a search for 'кварц'
Кварц is a synonym of Quartz.
Quartz
A valid IMA mineral species - grandfathered
This page kindly sponsored by Dragon Minerals
About Quartz
Formula:
SiO2
Colour:
Colorless, purple, rose, red, black, yellow, brown, green, blue, orange, etc.
Lustre:
Vitreous
Hardness:
7
Specific Gravity:
2.65 - 2.66
Crystal System:
Trigonal
Name:
Quartz has been known and appreciated since pre-historic times. The most ancient name known is recorded by Theophrastus in about 300-325 BCE, κρύσταλλος or kristallos. The varietal names, rock crystal and bergcrystal, preserve the ancient usage. The root words κρύοσ signifying ice cold and στέλλειυ to contract (or solidify) suggest the ancient belief that kristallos was permanently solidified ice.
The earliest printed use of "querz" was anonymously published in 1505, but attributed to a physician in Freiberg, Germany, Ulrich Rülein von Kalbe (a.k.a. Rülein von Calw, 1527). Agricola used the spelling "quarzum" (Agricola 1530) as well as "querze", but Agricola also referred to "crystallum", "silicum", "silex", and silice". Tomkeieff (1941) suggested an etymology for quartz: "The Saxon miners called large veins - Gänge, and the small cross veins or stringers - Querklüfte. The name ore (Erz, Ertz) was applied to the metallic minerals, the gangue or to the vein material as a whole. In the Erzgebirge, silver ore is frequently found in small cross veins composed of silica. It may be that this ore was called by the Saxon miners 'Querkluftertz' or the cross-vein-ore. Such a clumsy word as 'Querkluftertz' could easily be condensed to 'Querertz' and then to 'Quertz', and eventually become 'Quarz' in German, 'quarzum' in Latin and 'quartz' in English." Tomkeieff (1941, q.v.) noted that "quarz", in its various spellings, was not used by other noted contemporary authors. "Quarz" was used in later literature referring to the Saxony mining district, but seldom elsewhere.
Gradually, there were more references to quartz: E. Brown in 1685 and Johan Gottschalk Wallerius in 1747. In 1669, Nicolaus Steno (Niels Steensen) obliquely formulated the concept of the constancy of interfacial angles in the caption of an illustration of quartz crystals. He referred to them as "cristallus" and "crystallus montium".
Tomkeieff (1941) also noted that Erasmus Bartholinus (1669) used the various spellings for "crystal" to signify other species than quartz and that crystal could refer to other "angulata corpora" (bodies with angles): "In any case in the second half of the XVIIIth century quartz became established as a name of a particular mineral and the name crystal became a generic term synonymous with the old term 'corpus angulatum'."
The earliest printed use of "querz" was anonymously published in 1505, but attributed to a physician in Freiberg, Germany, Ulrich Rülein von Kalbe (a.k.a. Rülein von Calw, 1527). Agricola used the spelling "quarzum" (Agricola 1530) as well as "querze", but Agricola also referred to "crystallum", "silicum", "silex", and silice". Tomkeieff (1941) suggested an etymology for quartz: "The Saxon miners called large veins - Gänge, and the small cross veins or stringers - Querklüfte. The name ore (Erz, Ertz) was applied to the metallic minerals, the gangue or to the vein material as a whole. In the Erzgebirge, silver ore is frequently found in small cross veins composed of silica. It may be that this ore was called by the Saxon miners 'Querkluftertz' or the cross-vein-ore. Such a clumsy word as 'Querkluftertz' could easily be condensed to 'Querertz' and then to 'Quertz', and eventually become 'Quarz' in German, 'quarzum' in Latin and 'quartz' in English." Tomkeieff (1941, q.v.) noted that "quarz", in its various spellings, was not used by other noted contemporary authors. "Quarz" was used in later literature referring to the Saxony mining district, but seldom elsewhere.
Gradually, there were more references to quartz: E. Brown in 1685 and Johan Gottschalk Wallerius in 1747. In 1669, Nicolaus Steno (Niels Steensen) obliquely formulated the concept of the constancy of interfacial angles in the caption of an illustration of quartz crystals. He referred to them as "cristallus" and "crystallus montium".
Tomkeieff (1941) also noted that Erasmus Bartholinus (1669) used the various spellings for "crystal" to signify other species than quartz and that crystal could refer to other "angulata corpora" (bodies with angles): "In any case in the second half of the XVIIIth century quartz became established as a name of a particular mineral and the name crystal became a generic term synonymous with the old term 'corpus angulatum'."
Polymorph of:
Isostructural with:
Quartz is one of the most common minerals found in the Earth's crust. If pure, quartz forms colorless, transparent and very hard crystals with a glass-like luster. A significant component of many igneous, metamorphic and sedimentary rocks, this natural form of silicon dioxide is found in an impressive range of varieties and colours.
The Si analogue of pertoldite.
Quartz occurs in two basic forms:
1. The more common macrocrystalline quartz is made of visible crystals or grains. Examples are rock crystals, the grains in sandstone, but also massive quartz that is made of large crystallites without any crystal faces, like vein quartz.
2. Cryptocrystalline quartz or microcrystalline quartz is made of dense and compact aggregates of microscopic quartz crystals and crystallites. Examples are agate and chert. The different types of cryptocrystalline quartz are colloquially subsumed under the term chalcedony, although that term has a more strict definition in scientific literature. It is worth mentioning that most chalcedony contains small amounts of another SiO2 polymorph, moganite, so it is not always pure quartz.
Quartz crystals or aggregates that share certain peculiar physical properties have been classified as quartz varieties with specific "trivial names".
The best known examples are the colored varieties of quartz, like amethyst or smoky quartz, but there are also trivial names for specific crystal shapes, aggregates and textures, like scepter quartz, gwindel or quartzine. Because there are no canonical rules on naming or defining quartz varieties like they are for minerals, the definitions of some quartz varieties are precise and generally accepted, while the definitions of others vary considerably between different authors, or are rather fuzzy.
Mindat Classification of Quartz Varieties
On Mindat, macrocrystalline quartz and its varieties are listed as quartz and varieties of quartz.
Cryptocrystalline quartz and its varieties are listed as chalcedony, like "Quartz (Var: Chalcedony)", or as variety of chalcedony, like "Chalcedony (Var: Agate)".
More about the specific properties of chalcedony and its varieties can be found at the respective mineral pages.
Note that, contrary to minerals, the definitions of varieties are not mutually exclusive in the sense that no mineral can be another. A single specimen can be correctly classified as several varieties.
Quartz can be thought of as being made of threefold and sixfold helical chains of SiO4 tetrahedra that run parallel to the c axis. Figure 1 shows two representations of a threefold SiO4 helix and its relationship to the quartz unit cell: to the right a ball model with red oxygen and white silicon atoms, to the left a tetrahedral model, with the corners of the tetrahedra at the position of the oxygen atoms.
Six of such helices are connected to form a ring that surrounds a central channel which runs parallel to the c-axis, sometimes called "c-channel". The SiO4 tetrahedra around the central c-channel form two independent sixfold helices. Figure 2 shows two views of the corresponding structure: looking in the direction of the c-axis in the top row, and looking in the direction of an a-axis in the bottom row. Like quartz crystals, the ring is six-sided but has a trigonal symmetry. The large channels are an important structural feature of quartz because they may be occupied by small cations.
You can explore the crystal structure of quartz with the interactive tool JSmol further down this page.
A helix is either turning clockwise (right-handed) or counter-clockwise (left-handed). Due to the helical arrangement of the SiO4 tetrahedra, the atomic lattice of quartz possesses the symmetry properties of a helix: Quartz forms left- and right-handed crystals, whose crystal structure and morphology are mirror-images of each other.
In a crystal with space group P3121 (right), the sixfold helices turn counter-clockwise (left) and the threefold helices clockwise (right).
In a crystal with space group P3221 (left), the sixfold helices turn clockwise (right) and the threefold helices counter-clockwise (left).
For a thorough review of the symmetry features of quartz, see Heaney (1994).
The crystallographic form of quartz that is characteristic for its symmetry properties is the trigonal trapezohedron. The position of the faces of the positive trigonal trapezohedra on the crystal reflects the handedness of the structure of the crystal. The figure to the right visualizes the relationship between the handedness of the six-fold helices and the position of the faces of the positive trigonal trapezohedron (x - orange) and the trigonal bipyramid (s - blue). Unfortunately, these faces are not present on all crystals, and often it is not possible to determine the handedness of a crystal from its morphology.
Quartz is optically active: the polarization of a light ray passing through a crystal parallel to the c-axis will be rotated either to the left or the right, depending on the handedness of the crystal (Arago, 1811; Biot, 1812; Herschel, 1822). The relationship between handedness of the crystals and the symmetry of the structure and hence the optical rotation was determined by de Vries (1958).
The following table lists how symmetry, morphology and optical behaviour are related.
Note that the morphological handedness as expressed by the position of the trapezohedral and bipyramidal faces x and s does not match the symmetry's handedness:
Quartz is found as individual crystals and as crystal aggregates. Well crystallized quartz crystals are typically six-sided prisms with steep pyramidal terminations. They can be stubby ("short prismatic") or elongated and even needle-like. In most environments quartz crystals are attached to the host rock and only have one tip, but double-terminated crystals are also found.
As a rock-forming mineral, quartz commonly occurs as sub-millimeter to centimeter-sized anhedral grains, well-formed crystals are uncommon. Secondary vein-fillings of quartz are typically massive.
Quartz belongs to the trigonal-trapezohedral crystal class 32. Of the seven basic crystallographic forms of this crystal class, the hexagonal prism and trigonal rhombohedra are very common and determine the overall shape of the crystals. The trigonal bipyramids and trigonal trapezohedra are frequently found, but typically only as relatively small faces. The trigonal prisms, the basal pinacoid and in particular ditrigonal prisms are very rare (Frondel, 1962).
Quartz crystals show about 100 different crystallographic forms in nature (Frondel, 1962; Rykart, 1995). It is convenient and common practice to designate them with Latin and Greek letter symbols instead of Miller-Bravais indices. The following figure illustrates the relation of the common forms (sorted by abundance) to the faces found on quartz crystals. The most common combination of crystallographic forms in quartz crystals is r+m+z.
The handedness of quartz crystals can be determined easily from the positions of x faces, which are at the lower left or lower right corner of the r face (orange faces in Fig.5). With some difficulty the handedness can be determined from the position of the s faces (blue faces in Fig.5), which lie between the r and z faces: the s face often shows a fine striation that runs parallel to the edge of the r-face.
The bottom row shows a top view of the crystals. It does not only show their trigonal symmetry but also the chirality of the position of the x faces.
Macroscopic Structure of Quartz Crystals
In response to lattice defects, and apparently reflecting their growth conditions, quartz crystals may develop two very distinct and mutually exclusive types of internal structure:
- Macromosaic Structure, sometimes called "Friedlaender Quartz"
- Lamellar Structure, sometimes called "Bambauer Quartz"
Individual crystals may possess both structural types, but the respective parts of the crystals grew at different developmental stages (Hertweck et al., 1998). It is sometimes claimed that all quartz occurs either as macromosaic or as lamellar structural type. This is not correct.
The lamellar structure was first described by Weil (1931). The crystals contain layers that show an optical anomaly: they are biaxial. The layers are stacked parallel to the crystal faces in an onion-like manner and were found to be associated with a relatively high hydrogen and aluminium content (Bambauer et al., 1961, 1962, 1963). Lamellar quartz cannot be safely recognized without studying the optical properties of the crystal in a thin section.
Macromosaic quartz crystals have been described by Friedlaender (1951) and are composed of slightly tilted and radially arranged wedge-shaped sectors. They are recognized by the presence of sutures on the crystal faces which are often confused with twin boundaries. Crystals with such a structure are found in pegmatite and miarole pockets and in high-temperature alpine-type fissures.
Quartz Crystal Habits
Strictly speaking, the term "habit" is used to designate the overall shape of individual crystals, regardless of the crystallographic forms (crystal faces) involved. Confusingly, the definitions of some habits of quartz crystals do include specific forms. Many of the trivial names of these habits have been introduced and popularized by rock hounds in the Alps (for a good overview, see Rykart, 1995). The most important habits with trivial names (with synonyms in different languages in braces) are:
a) Normal habit ("Maderaner Habitus", prismatic habit): "typical" quartz crystals that are not or only slightly tapered.
b) Trigonal habit: Crystals with obvious trigonal symmetry, for example, because of missing z faces, or because of a triangular cross section, like in crystals with a Muzo habit (h).
c) Pseudohexagonal habit: Crystals with an even development of rhombohedral and prism faces.
d) Cumberland habit: Crystals with very small or absent prism faces, often bipyramidal.
e) Pseudocubic quartz (pseudocubic habit, cubic habit, cube quartz, "Würfelquarz"): Crystals with a dominant r or z form that look like slightly distorted cubes.
f) Dauphiné habit: Crystal tips with a single very dominant rhombohedral face.
g) Tessin habit ("Abito Ticino", "Tessiner Habitus", "Rauriser Habitus", "Penninischer Habitus", "Acute Rhombohedral Habit"): Crystals that are tapered by steep rhombohedral faces { h 0 i 1 }, Tessin habit in the strict sense is dominated by { 4 0 4 1 } and { 3 0 3 1 } faces. At the original locality, they possess a macromosaic structure.
h) Muzo habit: Crystals with prism faces that are tapered under the z faces because these are made of a succession of alternating m and z faces, and who have a trigonal cross section at the crystal tips (Gansser, 1963).
Needle quartz (acicular habit): Crystals greatly elongated along the c-axis.
Quartz Growth Forms
In addition to crystallographic forms and habits, many quartz crystals are characterized by peculiar morphological features that reflect different modes of growth during their development. Some of these "growth forms" are found at many different localities and - like habits - have been given "trivial names" (e.g., "cactus quartz", "gwindel"). Some of these are listed as varieties of quartz on Mindat. Among the more common and important growth forms are:
Sceptre quartz: Crystals with syntaxial overgrowth of a second generation tip.
Faden quartz: Crystals and crystal aggregates with a white thread running through the crystals. The thread is caused by repetitive cracking of the crystal during growth and consists of fluid inclusions.
Window or Skeleton or Frame or Fenster quartz: Crystals with frame-like, elevated edges of the crystal faces, usually with parallel grown blades that grow from the edges to the center of the faces in a window glass-like manner. Hopper crystals that correspond to skeleton-growth in the strict sense are rare.
Phantom quartz: Crystals in which outlines of the shape of earlier developmental stages of the crystal are visible because of inclusions or color zones.
Sprouting quartz ("Sprossenquarz"): Crystals on which split-growth causes subparallel daughter crystals to sprout from the crystal faces
Artichoke quartz: A form of split-growth resulting in specimens with composite artichoke-like crystal tips.
Gwindel: Crystals elongated and twisted along an a-axis.
Cactus quartz or spirit quartz: Crystals whose prism faces are covered by small, roughly radially grown second-generation crystals.
Quartz Twins
Twinning is very common in quartz, but is often inconspicuous and difficult to recognize. Two types of twinning can be distinguished (data in tables from Jentzsch, 1867, 1868; Gault, 1949; Frondel, 1962):
1. Twins with parallel main crystallographic axes
Dauphiné and Brazil law twins are very common. Most crystals, even if morphologically untwinned, contain at least small twin domains. Both types of twins can be found in a single crystal.
Dauphiné Law
Also called: Swiss Law, Alpine Law
Dauphiné law twins can be thought of as a merger of two crystals of equal handedness that are rotated by 60° around the c-axis relative to each other (Weiss, 1816). They are penetration twins composed of twin domains with irregular boundaries (Leydolt, 1855). The size and shape of the twin domains can vary and the shares of the twin domains in a crystal do not have to be equal. The degree of intergrowth of the domains may increase during growth, starting from roughly triangular sectors at the base to complex irregular patterns at the tip of the crystal (Friedlaender, 1951). Twin domains are only rarely visible in natural crystals and normally need to get visualized by etching the surface or a polished cross-section (Leydolt, 1855; Judd, 1888). Electron microscopical studies reveal that on a small scale the twin domains look like complex polygons with straight boundaries (Lang, 1965; McLaren and Phakey, 1969).
Dauphiné twins can sometimes be recognised by the position and arrangement of crystal faces, in particular, the x-faces. Because the rhombohedral faces are composites of r and z faces, they do not show the common size difference of the faces and the crystals assume a pseudohexagonal habit.
Rarely Dauphiné twinned crystals that lack one type of rhombohedral face (either r or z) - and that would display a trigonal habit if they were untwinned - show re-entrant angles at the tip that make them look like drill heads (for example, Schäfer, 1999).
Dauphiné twins are sometimes called electrical twins, because this kind of twinning reduces or even suppresses the piezoelectricity that is typical for untwinned quartz crystals, while their optical activity remains unaffected (Thomas, 1945; Donnay and Le Page, 1975).
Brazil Law
Also called: Optical Law
Brazil law twins can be thought of as a merger of a left- and right-handed crystal: they are penetration twins composed of left- and right-handed domains. Their twin boundaries are usually straight lines, resulting in a characteristic pattern made of straight lines and triangles (Leydolt, 1855). As with Dauphiné twins, the twin domains are usually not visible in natural crystals and need to be visualized by etching (Leydolt, 1855). The corresponding surface patterns on crystal faces are polygonal patches with straight boundaries, often triangular.
Brazil law twins that show the ideal arrangement of x and s crystal faces are very rare.
Many amethysts are twinned polysynthetically according to the Brazil Law: Parts of the amethyst crystals, in particular in zones under the r rhombohedral faces are composed of alternating layers of left- and right-handed quartz (Brewster 1823; McLaren and Pitkethly, 1982; Taijing and Sunagawa, 1990). The gauge of individual layers is normally less than 1 mm. The layered structure may be visible as a fingerprint-like pattern on rhombohedral faces.
Brazil law twins are sometimes called optical twins, because this kind of twinning reduces or even suppresses the optical activity typical for quartz crystals. Confusingly, and contrary to common belief, Brazil law twinning does also reduce or suppress the piezoelectricity of quartz crystals (Thomas, 1945; Donnay and Le Page, 1975).
Combined Law
Also called: Liebisch Law, Dauphiné-Brazil Law, Leydolt Law
It is not unusual for crystals to show Dauphiné and Brazil law domains in one crystal, and sometimes crystals show x or s faces at positions that would indicate a special type of twinning. Electron microscopic studies show that when Brazil law twins are heated and develop new Dauphiné twin domains, their left- and right-handed domains do not share boundaries when they are rotated with respect to each other (Van Goethem et al., 1977), so Liebisch twinning seems to be energetically less favorable. Accordingly, Liebisch twinning is rare.
2. Twins with inclined main crystallographic axes (incomplete list)
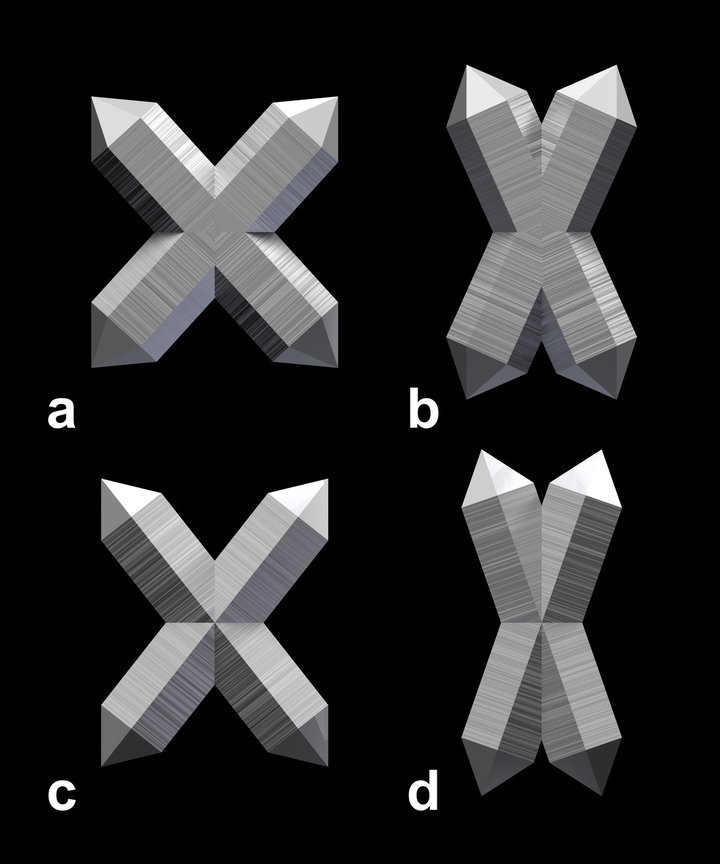 Of the twins with inclined main axes, only the Japan law twin is common and well established, while for some of the others (including some that are not listed here) only a few and sometimes only one specimen have been reported and the existence of a twin law is questionable. The Reichenstein-Grieserntal Law is sometimes erroneously called "Esterel Law", which is the equivalent for beta-quartz.
Of the twins with inclined main axes, only the Japan law twin is common and well established, while for some of the others (including some that are not listed here) only a few and sometimes only one specimen have been reported and the existence of a twin law is questionable. The Reichenstein-Grieserntal Law is sometimes erroneously called "Esterel Law", which is the equivalent for beta-quartz.
Japan Law
Also called: Weiss Law, La Gardette Law
Japan law twins are the only common quartz twins with inclined c axes. The law was first found and described by Weiss (1829) on crystals from La Gardette, France, but the name "Japan law" became more popular after a great number of them were found in Japan. The c-axis of two crystals meet at an angle of 84°33', with two of the m prism faces of both crystals being parallel. The twinning plane {1 1 2 2} of Japan law twins corresponds to the flat trigonal bipyramid ξ (the Greek letter xi).
Japan law twins are contact twins (Sunagawa and Yasuda, 1983). The twin junctions often look jagged on the crystal surface, but are perfectly straight in the interior of the crystals, and form a thin plane that runs from the base of the crystal to the V-shaped indentation between the branches (Sunagawa and Yasuda, 1983). Electron microscopic studies revealed that the twin boundary also forms a perfect plane parallel to {1 1 2 2} (Lenart et al. 2012; Momma et al. 2015), but appears to be restricted to the initial growth periods of the crystal, extending only a few hundred micrometers, which has been interpreted as an indication of a formation as a nucleation twin (Lenart et al. 2012). The cause of the twin formation is still not understood.
Most Japan law twins are flattened, and often they are larger than untwinned crystals that accompany them. Depending on the handedness of the two branches of a twin, one can distinguish 8 different basic twinning subtypes that are also twinned according to the Brazil or Dauphiné law (Frondel, 1962), but the pattern of Brazil and Dauphiné twin domains can be very complex (Kozu, 1952).
Compared to many other minerals, quartz is chemically very pure, most crystals contain more than 99.5% SiO2. Nevertheless, varieties colored by impurities occur. These can be devided into two groups:
1. Quartz colored by trace elements built into the crystal lattice.
Only a few elements can replace silicon in the quartz lattice (substitutional positions) or are small enough to occupy free spaces in the lattice (interstitial positions). In natural quartz crystals, the most common ones to replace Si are Al, Fe, Ge, and Ti, whereas Li, Na, Ca, Mg and Fe often occupy interstitial positions in the "c-channels" mentioned under "Structure of Quartz". Of the substitutional trace elements, only Al, Fe and more rarely P are found to play a role in natural colored varieties. There are only a handful of quartz varieties colored by trace elements built into the lattice, sorted by abundance, with the more common ones first:
- Smoky quartz
- Amethyst
- Citrine
- Pink Quartz / Euhedral Rose Quartz
- Prasiolite
With the possible exception of some prasiolites and some citrines, the color of these varieties is based on color centers whose formation requires high energy irradiation from radioactive elements in the surrounding rocks (O'Brien, 1955; Lehmann and Moore, 1966; Maschmeyer et al., 1980; Maschmeier and Lehmann, 1983). Quartz varieties based on color centers are pleochroic, and their color centers can be destroyed by heat treatment.
Note that individual quartz crystals may contain several colored varieties, like an amethyst with smoky zones.
2. Quartz colored by inclusions of separate phases, for example minerals or fluids.
Because quartz crystals grow in many geological environments, they embed many different minerals during growth and assume the colors of the included minerals. Colors may also be caused by light scattering at finely distributed but colorless inclusions.
There are also trivial names for varieties colored by inclusions that have been found at many localities, like "prase", "ferruginous quartz" or "rose quartz". However, the definitions of these varieties are often rather fuzzy, and different authors use different definitions.
Quartz is one of the crystalline forms of silica, the essential building material for all silicates, and quartz can only form where silica is present in excess of what is consumed in the formation of other silicate minerals.
Quartz may also be consumed during the formation of new silicate minerals, in particular at higher temperatures and pressures, and certain geological environments are "incompatible" with free silica and hence quartz.
Quartz as a Rock-Forming Mineral
Silica has been enriched in the continental Earth's crust to about 60% (Rudnick and Gao, 2003) by processes like magmatic differentiation and the formation of silica-rich igneous rocks (mainly driven by plate tectonics) and the accumulation of the physically and chemically stable quartz in sediments and sedimentary rocks. The oceanic crust's silica content of about 50% (White and Klein, 2014) in its igneous rocks is too low for quartz to form in them.
The largest amount of quartz is found as a rock-forming mineral in silica-rich igneous rocks, namely granite-like plutonic rocks, and in the metamorphic rocks that are derived from them. Under conditions at or near the surface, quartz is generally more stable than most other rock-forming minerals and its accumulation in sediments leads to rocks that are highly enriched in quartz, like sandstones. Quartz is also a major constituent of sedimentary rocks whose high quartz content is not immediately obvious, like slates, as well as in the metamorphic rocks derived from such quartz-bearing precursor rocks.
Quartz Veins
At higher temperatures and pressures quartz is easily dissolved by watery fluids percolating the rock. When silica-rich solutions penetrate cooler rocks, the silica will precipitate as quartz in fissures, forming thin white seams as well as large veins which may extend over many kilometers (Bons, 2001; Wangen and Munz, 2004, Pati et al, 2007). In most cases, the quartz in these veins will be massive, but they may also contain well-formed quartz crystals. Phyllites and schists often contain thin lenticular or regular veins of so-called "segregation quartz" (Vinx, 2013) that run parallel to the bedding and are the result of local transport of silica during metamorphosis (Chapman, 1950; Sawyer and Robin, 1986). Silica-rich fluids are also driven out of solidifying magma bodies. When these hot brines enter cooler rocks, the solution gets oversaturated in silica, and quartz forms.
Along with the silica, metals are also transported with the brines and precipitate in the veins as sometimes valuable ore minerals. The association of gold and quartz veins is a well-known example. Quartz is the most common "gangue mineral" in ore deposits.
Quartz Crystals
Quartz crystals typically grow in fluids at elevated temperatures between 150°C and 600°C, but they also grow at ambient conditions (Mackenzie and Gees, 1971; Ries and Menckhoff, 2008).
Quartz is best known for the beautiful crystals it forms in all sorts of cavities and fissures. The greatest variety of shapes and colors of quartz crystals comes from hydrothermal ore veins and deposits, reflecting large differences in growth conditions in these environments (chemistry, temperature, pressure). Splendid, large crystals grow from ascending hot brines in large fissures, from residual silica-rich fluids in cavities in pegmatites and from locally mobilized silica in Alpine-type fissures. An economically important source of amethyst for the lapidary industry are cavities of volcanic rocks. Small, but well-formed quartz crystals are found in septarian nodules, and in dissolution pockets in limestones.
Well-formed quartz crystals that are fully embedded in sedimentary rocks and grew during diagenesis (so-called authigenic quartz crystals) are occasionally found in limestones, marls, and evaporites (e.g. Rykart, 1984).
Euhedral quartz crystals that are embedded in igneous rocks are uncommon. Quartz is among the last minerals that form during the solidification of a magma, and because the crystals fill the residual space between the older crystals of other minerals they are usually irregular. Euhedral, stubby bipyramidal quartz crystals are occasionally found in rhyolites. These are usually paramorphs after beta-quartz with hexagonal symmetry, quartz crystals whose trigonal habit shows that they grew as alpha-quartz are very rare in volcanic rocks (e.g. Flick and Weissenbach, 1978). Only rarely are euhedral quartz crystals seen embedded in metamorphic rocks (Kenngott, 1854; Tschermak, 1874; Heddle, 1901).
In most cases quartz is easy to identify by its combination of the following properties:
- hardness (easily scratches glass, also harder than steel)
- glass-like luster
- poor to indistinct cleavage
- conchoidal fracture in crystals, in massive specimens the fracture often looks irregular to the naked eye, but still conchoidal at high magnification.
Note that in macrocrystalline quartz the fracture surfaces have a vitreous to resinous luster, whereas in cryptocrystalline quartz (chalcedony) fractured surfaces are dull.
Crystals are very common and their usually six-sided shape and six-sided pyramidal tips are well-known. Intergrown crystals without tips can often be recognized by the presence of the characteristic striation on the prism faces.
Quartz as a rock-forming mineral, in particular as irregular grains in the matrix, occasionally poses problems and may require additional means of identification. It may be confused with cordierite (pleochroic, tendency to alteration) and nepheline (lower hardness, geological environment incompatible with quartz).
In thin sections macrocrystalline quartz appears clear and homogeneous, with blue-gray to white or bright yellow interference colors and a low relief. Quartz does not show alterations at grain boundaries. Strained quartz grains from metamorphic rocks show a so-called "undulatory extinction" (Blatt and Christie, 1963).
Quartz is one of the few minerals on Mindat where "visual identification" may be accepted as a method of identification for new locality entries and photos of well-formed crystals. In other cases, at least hardness should be checked, too.
For quartz as a rock-forming mineral visual identification is often insufficient.
Quartz normally does not require special attention when handled or stored. At ambient conditions, quartz is chemically almost inert, so it does not suffer from the problems seen in many other minerals. Crystals do not disintegrate or crumble, they do not oxidize or dissolve easily in water and they don't mind being touched. The only problem for the collector is dust, which will find and cover your crystals, no matter what you do.
Quartz crystals that contain large fluid or gas inclusions may crack when heated - skeleton quartz is the most sensitive variety in this respect - but most quartz specimens can take some heat, like cleaning in warm water, without being damaged.
Quartz is hard but quite brittle, and with some effort, one can damage a crystal even with things that are much softer. The edges of the crystals are very often slightly damaged because crystals were not kept separate from each other.
Colored quartz varieties can pale in sunlight, the most sensitive variety is euhedral rose quartz/pink quartz, which should be kept in the dark. Amethyst, smoky quartz and natural citrine will also pale, but it takes very long.
Mild ultrasonic cleaning is usually not a problem as long the crystals are not internally cracked, but some varieties may be damaged, in particular, amethyst (due to its polysynthetic Brazil law twinning) and skeleton quartz with liquid and gas inclusions.
Rock Currier wrote a Mindat article on cleaning quartz that is worthwhile reading: http://www.mindat.org/article.php/403/Cleaning+Quartz
When cutting, grinding and polishing specimens, keep in mind that quartz dust will cause silicosis (for a review, see Goldsmith, 1994), do not cut or grind dry and wear an appropriate dust mask.
Quartz bear, on average, 10 ppmw (5 ppmw median) of water. Crystals rich in OH defects may bear as much as 250 ppmw (maximum).
 Visit gemdat.org for gemological information about Quartz.
Visit gemdat.org for gemological information about Quartz.
The Si analogue of pertoldite.
Macro- and Cryptocrystalline Quartz
Quartz occurs in two basic forms:
1. The more common macrocrystalline quartz is made of visible crystals or grains. Examples are rock crystals, the grains in sandstone, but also massive quartz that is made of large crystallites without any crystal faces, like vein quartz.
2. Cryptocrystalline quartz or microcrystalline quartz is made of dense and compact aggregates of microscopic quartz crystals and crystallites. Examples are agate and chert. The different types of cryptocrystalline quartz are colloquially subsumed under the term chalcedony, although that term has a more strict definition in scientific literature. It is worth mentioning that most chalcedony contains small amounts of another SiO2 polymorph, moganite, so it is not always pure quartz.
Quartz Varieties
Quartz crystals or aggregates that share certain peculiar physical properties have been classified as quartz varieties with specific "trivial names".
The best known examples are the colored varieties of quartz, like amethyst or smoky quartz, but there are also trivial names for specific crystal shapes, aggregates and textures, like scepter quartz, gwindel or quartzine. Because there are no canonical rules on naming or defining quartz varieties like they are for minerals, the definitions of some quartz varieties are precise and generally accepted, while the definitions of others vary considerably between different authors, or are rather fuzzy.
Mindat Classification of Quartz Varieties
On Mindat, macrocrystalline quartz and its varieties are listed as quartz and varieties of quartz.
Cryptocrystalline quartz and its varieties are listed as chalcedony, like "Quartz (Var: Chalcedony)", or as variety of chalcedony, like "Chalcedony (Var: Agate)".
More about the specific properties of chalcedony and its varieties can be found at the respective mineral pages.
Note that, contrary to minerals, the definitions of varieties are not mutually exclusive in the sense that no mineral can be another. A single specimen can be correctly classified as several varieties.
Structure of Quartz
The structure of quartz was deciphered by Bragg and Gibbs in 1925 (for a review of the structure and symmetry features of quartz, see Heaney, 1994). Its basic building block is the SiO4 group, in which four oxygen atoms surround a central silicon atom to form a tetrahedron. Since each oxygen is member of two SiO4 groups, the formula of quartz is SiO2. The SiO4 tetrahedra form a three-dimensional network and many mineralogy textbooks classify quartz as a network silicate or tectosilicate.Quartz can be thought of as being made of threefold and sixfold helical chains of SiO4 tetrahedra that run parallel to the c axis. Figure 1 shows two representations of a threefold SiO4 helix and its relationship to the quartz unit cell: to the right a ball model with red oxygen and white silicon atoms, to the left a tetrahedral model, with the corners of the tetrahedra at the position of the oxygen atoms.
Six of such helices are connected to form a ring that surrounds a central channel which runs parallel to the c-axis, sometimes called "c-channel". The SiO4 tetrahedra around the central c-channel form two independent sixfold helices. Figure 2 shows two views of the corresponding structure: looking in the direction of the c-axis in the top row, and looking in the direction of an a-axis in the bottom row. Like quartz crystals, the ring is six-sided but has a trigonal symmetry. The large channels are an important structural feature of quartz because they may be occupied by small cations.
You can explore the crystal structure of quartz with the interactive tool JSmol further down this page.
Handedness of Quartz Crystals
A helix is either turning clockwise (right-handed) or counter-clockwise (left-handed). Due to the helical arrangement of the SiO4 tetrahedra, the atomic lattice of quartz possesses the symmetry properties of a helix: Quartz forms left- and right-handed crystals, whose crystal structure and morphology are mirror-images of each other.
In a crystal with space group P3121 (right), the sixfold helices turn counter-clockwise (left) and the threefold helices clockwise (right).
In a crystal with space group P3221 (left), the sixfold helices turn clockwise (right) and the threefold helices counter-clockwise (left).
For a thorough review of the symmetry features of quartz, see Heaney (1994).
The crystallographic form of quartz that is characteristic for its symmetry properties is the trigonal trapezohedron. The position of the faces of the positive trigonal trapezohedra on the crystal reflects the handedness of the structure of the crystal. The figure to the right visualizes the relationship between the handedness of the six-fold helices and the position of the faces of the positive trigonal trapezohedron (x - orange) and the trigonal bipyramid (s - blue). Unfortunately, these faces are not present on all crystals, and often it is not possible to determine the handedness of a crystal from its morphology.
Quartz is optically active: the polarization of a light ray passing through a crystal parallel to the c-axis will be rotated either to the left or the right, depending on the handedness of the crystal (Arago, 1811; Biot, 1812; Herschel, 1822). The relationship between handedness of the crystals and the symmetry of the structure and hence the optical rotation was determined by de Vries (1958).
The following table lists how symmetry, morphology and optical behaviour are related.
Note that the morphological handedness as expressed by the position of the trapezohedral and bipyramidal faces x and s does not match the symmetry's handedness:
|
Morphology
Quartz is found as individual crystals and as crystal aggregates. Well crystallized quartz crystals are typically six-sided prisms with steep pyramidal terminations. They can be stubby ("short prismatic") or elongated and even needle-like. In most environments quartz crystals are attached to the host rock and only have one tip, but double-terminated crystals are also found.
As a rock-forming mineral, quartz commonly occurs as sub-millimeter to centimeter-sized anhedral grains, well-formed crystals are uncommon. Secondary vein-fillings of quartz are typically massive.
Quartz belongs to the trigonal-trapezohedral crystal class 32. Of the seven basic crystallographic forms of this crystal class, the hexagonal prism and trigonal rhombohedra are very common and determine the overall shape of the crystals. The trigonal bipyramids and trigonal trapezohedra are frequently found, but typically only as relatively small faces. The trigonal prisms, the basal pinacoid and in particular ditrigonal prisms are very rare (Frondel, 1962).
Quartz crystals show about 100 different crystallographic forms in nature (Frondel, 1962; Rykart, 1995). It is convenient and common practice to designate them with Latin and Greek letter symbols instead of Miller-Bravais indices. The following figure illustrates the relation of the common forms (sorted by abundance) to the faces found on quartz crystals. The most common combination of crystallographic forms in quartz crystals is r+m+z.
The handedness of quartz crystals can be determined easily from the positions of x faces, which are at the lower left or lower right corner of the r face (orange faces in Fig.5). With some difficulty the handedness can be determined from the position of the s faces (blue faces in Fig.5), which lie between the r and z faces: the s face often shows a fine striation that runs parallel to the edge of the r-face.
The bottom row shows a top view of the crystals. It does not only show their trigonal symmetry but also the chirality of the position of the x faces.
Macroscopic Structure of Quartz Crystals
In response to lattice defects, and apparently reflecting their growth conditions, quartz crystals may develop two very distinct and mutually exclusive types of internal structure:
- Macromosaic Structure, sometimes called "Friedlaender Quartz"
- Lamellar Structure, sometimes called "Bambauer Quartz"
Individual crystals may possess both structural types, but the respective parts of the crystals grew at different developmental stages (Hertweck et al., 1998). It is sometimes claimed that all quartz occurs either as macromosaic or as lamellar structural type. This is not correct.
The lamellar structure was first described by Weil (1931). The crystals contain layers that show an optical anomaly: they are biaxial. The layers are stacked parallel to the crystal faces in an onion-like manner and were found to be associated with a relatively high hydrogen and aluminium content (Bambauer et al., 1961, 1962, 1963). Lamellar quartz cannot be safely recognized without studying the optical properties of the crystal in a thin section.
Macromosaic quartz crystals have been described by Friedlaender (1951) and are composed of slightly tilted and radially arranged wedge-shaped sectors. They are recognized by the presence of sutures on the crystal faces which are often confused with twin boundaries. Crystals with such a structure are found in pegmatite and miarole pockets and in high-temperature alpine-type fissures.
Quartz Crystal Habits
Strictly speaking, the term "habit" is used to designate the overall shape of individual crystals, regardless of the crystallographic forms (crystal faces) involved. Confusingly, the definitions of some habits of quartz crystals do include specific forms. Many of the trivial names of these habits have been introduced and popularized by rock hounds in the Alps (for a good overview, see Rykart, 1995). The most important habits with trivial names (with synonyms in different languages in braces) are:
a) Normal habit ("Maderaner Habitus", prismatic habit): "typical" quartz crystals that are not or only slightly tapered.
b) Trigonal habit: Crystals with obvious trigonal symmetry, for example, because of missing z faces, or because of a triangular cross section, like in crystals with a Muzo habit (h).
c) Pseudohexagonal habit: Crystals with an even development of rhombohedral and prism faces.
d) Cumberland habit: Crystals with very small or absent prism faces, often bipyramidal.
e) Pseudocubic quartz (pseudocubic habit, cubic habit, cube quartz, "Würfelquarz"): Crystals with a dominant r or z form that look like slightly distorted cubes.
f) Dauphiné habit: Crystal tips with a single very dominant rhombohedral face.
g) Tessin habit ("Abito Ticino", "Tessiner Habitus", "Rauriser Habitus", "Penninischer Habitus", "Acute Rhombohedral Habit"): Crystals that are tapered by steep rhombohedral faces { h 0 i 1 }, Tessin habit in the strict sense is dominated by { 4 0 4 1 } and { 3 0 3 1 } faces. At the original locality, they possess a macromosaic structure.
h) Muzo habit: Crystals with prism faces that are tapered under the z faces because these are made of a succession of alternating m and z faces, and who have a trigonal cross section at the crystal tips (Gansser, 1963).
Needle quartz (acicular habit): Crystals greatly elongated along the c-axis.
Quartz Growth Forms
In addition to crystallographic forms and habits, many quartz crystals are characterized by peculiar morphological features that reflect different modes of growth during their development. Some of these "growth forms" are found at many different localities and - like habits - have been given "trivial names" (e.g., "cactus quartz", "gwindel"). Some of these are listed as varieties of quartz on Mindat. Among the more common and important growth forms are:
Sceptre quartz: Crystals with syntaxial overgrowth of a second generation tip.
Faden quartz: Crystals and crystal aggregates with a white thread running through the crystals. The thread is caused by repetitive cracking of the crystal during growth and consists of fluid inclusions.
Window or Skeleton or Frame or Fenster quartz: Crystals with frame-like, elevated edges of the crystal faces, usually with parallel grown blades that grow from the edges to the center of the faces in a window glass-like manner. Hopper crystals that correspond to skeleton-growth in the strict sense are rare.
Phantom quartz: Crystals in which outlines of the shape of earlier developmental stages of the crystal are visible because of inclusions or color zones.
Sprouting quartz ("Sprossenquarz"): Crystals on which split-growth causes subparallel daughter crystals to sprout from the crystal faces
Artichoke quartz: A form of split-growth resulting in specimens with composite artichoke-like crystal tips.
Gwindel: Crystals elongated and twisted along an a-axis.
Cactus quartz or spirit quartz: Crystals whose prism faces are covered by small, roughly radially grown second-generation crystals.
Quartz Twins
Twinning is very common in quartz, but is often inconspicuous and difficult to recognize. Two types of twinning can be distinguished (data in tables from Jentzsch, 1867, 1868; Gault, 1949; Frondel, 1962):
1. Twins with parallel main crystallographic axes
|
Dauphiné and Brazil law twins are very common. Most crystals, even if morphologically untwinned, contain at least small twin domains. Both types of twins can be found in a single crystal.
Dauphiné Law
Also called: Swiss Law, Alpine Law
Dauphiné law twins can be thought of as a merger of two crystals of equal handedness that are rotated by 60° around the c-axis relative to each other (Weiss, 1816). They are penetration twins composed of twin domains with irregular boundaries (Leydolt, 1855). The size and shape of the twin domains can vary and the shares of the twin domains in a crystal do not have to be equal. The degree of intergrowth of the domains may increase during growth, starting from roughly triangular sectors at the base to complex irregular patterns at the tip of the crystal (Friedlaender, 1951). Twin domains are only rarely visible in natural crystals and normally need to get visualized by etching the surface or a polished cross-section (Leydolt, 1855; Judd, 1888). Electron microscopical studies reveal that on a small scale the twin domains look like complex polygons with straight boundaries (Lang, 1965; McLaren and Phakey, 1969).
Dauphiné twins can sometimes be recognised by the position and arrangement of crystal faces, in particular, the x-faces. Because the rhombohedral faces are composites of r and z faces, they do not show the common size difference of the faces and the crystals assume a pseudohexagonal habit.
Rarely Dauphiné twinned crystals that lack one type of rhombohedral face (either r or z) - and that would display a trigonal habit if they were untwinned - show re-entrant angles at the tip that make them look like drill heads (for example, Schäfer, 1999).
Dauphiné twins are sometimes called electrical twins, because this kind of twinning reduces or even suppresses the piezoelectricity that is typical for untwinned quartz crystals, while their optical activity remains unaffected (Thomas, 1945; Donnay and Le Page, 1975).
Brazil Law
Also called: Optical Law
Brazil law twins can be thought of as a merger of a left- and right-handed crystal: they are penetration twins composed of left- and right-handed domains. Their twin boundaries are usually straight lines, resulting in a characteristic pattern made of straight lines and triangles (Leydolt, 1855). As with Dauphiné twins, the twin domains are usually not visible in natural crystals and need to be visualized by etching (Leydolt, 1855). The corresponding surface patterns on crystal faces are polygonal patches with straight boundaries, often triangular.
Brazil law twins that show the ideal arrangement of x and s crystal faces are very rare.
Many amethysts are twinned polysynthetically according to the Brazil Law: Parts of the amethyst crystals, in particular in zones under the r rhombohedral faces are composed of alternating layers of left- and right-handed quartz (Brewster 1823; McLaren and Pitkethly, 1982; Taijing and Sunagawa, 1990). The gauge of individual layers is normally less than 1 mm. The layered structure may be visible as a fingerprint-like pattern on rhombohedral faces.
Brazil law twins are sometimes called optical twins, because this kind of twinning reduces or even suppresses the optical activity typical for quartz crystals. Confusingly, and contrary to common belief, Brazil law twinning does also reduce or suppress the piezoelectricity of quartz crystals (Thomas, 1945; Donnay and Le Page, 1975).
Combined Law
Also called: Liebisch Law, Dauphiné-Brazil Law, Leydolt Law
It is not unusual for crystals to show Dauphiné and Brazil law domains in one crystal, and sometimes crystals show x or s faces at positions that would indicate a special type of twinning. Electron microscopic studies show that when Brazil law twins are heated and develop new Dauphiné twin domains, their left- and right-handed domains do not share boundaries when they are rotated with respect to each other (Van Goethem et al., 1977), so Liebisch twinning seems to be energetically less favorable. Accordingly, Liebisch twinning is rare.
2. Twins with inclined main crystallographic axes (incomplete list)
|

Fig.9: Twins with Inclined Axes.
a) Japan Law
b) Breithaupt Law
c) Reichenstein-Grieserntal Law
d) Zinnwald Law
a) Japan Law
b) Breithaupt Law
c) Reichenstein-Grieserntal Law
d) Zinnwald Law
Japan Law
Also called: Weiss Law, La Gardette Law
Japan law twins are the only common quartz twins with inclined c axes. The law was first found and described by Weiss (1829) on crystals from La Gardette, France, but the name "Japan law" became more popular after a great number of them were found in Japan. The c-axis of two crystals meet at an angle of 84°33', with two of the m prism faces of both crystals being parallel. The twinning plane {1 1 2 2} of Japan law twins corresponds to the flat trigonal bipyramid ξ (the Greek letter xi).
Japan law twins are contact twins (Sunagawa and Yasuda, 1983). The twin junctions often look jagged on the crystal surface, but are perfectly straight in the interior of the crystals, and form a thin plane that runs from the base of the crystal to the V-shaped indentation between the branches (Sunagawa and Yasuda, 1983). Electron microscopic studies revealed that the twin boundary also forms a perfect plane parallel to {1 1 2 2} (Lenart et al. 2012; Momma et al. 2015), but appears to be restricted to the initial growth periods of the crystal, extending only a few hundred micrometers, which has been interpreted as an indication of a formation as a nucleation twin (Lenart et al. 2012). The cause of the twin formation is still not understood.
Most Japan law twins are flattened, and often they are larger than untwinned crystals that accompany them. Depending on the handedness of the two branches of a twin, one can distinguish 8 different basic twinning subtypes that are also twinned according to the Brazil or Dauphiné law (Frondel, 1962), but the pattern of Brazil and Dauphiné twin domains can be very complex (Kozu, 1952).
Colored Quartz Varieties
Compared to many other minerals, quartz is chemically very pure, most crystals contain more than 99.5% SiO2. Nevertheless, varieties colored by impurities occur. These can be devided into two groups:
1. Quartz colored by trace elements built into the crystal lattice.
Only a few elements can replace silicon in the quartz lattice (substitutional positions) or are small enough to occupy free spaces in the lattice (interstitial positions). In natural quartz crystals, the most common ones to replace Si are Al, Fe, Ge, and Ti, whereas Li, Na, Ca, Mg and Fe often occupy interstitial positions in the "c-channels" mentioned under "Structure of Quartz". Of the substitutional trace elements, only Al, Fe and more rarely P are found to play a role in natural colored varieties. There are only a handful of quartz varieties colored by trace elements built into the lattice, sorted by abundance, with the more common ones first:
- Smoky quartz
- Amethyst
- Citrine
- Pink Quartz / Euhedral Rose Quartz
- Prasiolite
With the possible exception of some prasiolites and some citrines, the color of these varieties is based on color centers whose formation requires high energy irradiation from radioactive elements in the surrounding rocks (O'Brien, 1955; Lehmann and Moore, 1966; Maschmeyer et al., 1980; Maschmeier and Lehmann, 1983). Quartz varieties based on color centers are pleochroic, and their color centers can be destroyed by heat treatment.
Note that individual quartz crystals may contain several colored varieties, like an amethyst with smoky zones.
2. Quartz colored by inclusions of separate phases, for example minerals or fluids.
Because quartz crystals grow in many geological environments, they embed many different minerals during growth and assume the colors of the included minerals. Colors may also be caused by light scattering at finely distributed but colorless inclusions.
There are also trivial names for varieties colored by inclusions that have been found at many localities, like "prase", "ferruginous quartz" or "rose quartz". However, the definitions of these varieties are often rather fuzzy, and different authors use different definitions.
Occurrence of Quartz
Quartz is one of the crystalline forms of silica, the essential building material for all silicates, and quartz can only form where silica is present in excess of what is consumed in the formation of other silicate minerals.
Quartz may also be consumed during the formation of new silicate minerals, in particular at higher temperatures and pressures, and certain geological environments are "incompatible" with free silica and hence quartz.
Quartz as a Rock-Forming Mineral
Silica has been enriched in the continental Earth's crust to about 60% (Rudnick and Gao, 2003) by processes like magmatic differentiation and the formation of silica-rich igneous rocks (mainly driven by plate tectonics) and the accumulation of the physically and chemically stable quartz in sediments and sedimentary rocks. The oceanic crust's silica content of about 50% (White and Klein, 2014) in its igneous rocks is too low for quartz to form in them.
The largest amount of quartz is found as a rock-forming mineral in silica-rich igneous rocks, namely granite-like plutonic rocks, and in the metamorphic rocks that are derived from them. Under conditions at or near the surface, quartz is generally more stable than most other rock-forming minerals and its accumulation in sediments leads to rocks that are highly enriched in quartz, like sandstones. Quartz is also a major constituent of sedimentary rocks whose high quartz content is not immediately obvious, like slates, as well as in the metamorphic rocks derived from such quartz-bearing precursor rocks.
Quartz Veins
At higher temperatures and pressures quartz is easily dissolved by watery fluids percolating the rock. When silica-rich solutions penetrate cooler rocks, the silica will precipitate as quartz in fissures, forming thin white seams as well as large veins which may extend over many kilometers (Bons, 2001; Wangen and Munz, 2004, Pati et al, 2007). In most cases, the quartz in these veins will be massive, but they may also contain well-formed quartz crystals. Phyllites and schists often contain thin lenticular or regular veins of so-called "segregation quartz" (Vinx, 2013) that run parallel to the bedding and are the result of local transport of silica during metamorphosis (Chapman, 1950; Sawyer and Robin, 1986). Silica-rich fluids are also driven out of solidifying magma bodies. When these hot brines enter cooler rocks, the solution gets oversaturated in silica, and quartz forms.
Along with the silica, metals are also transported with the brines and precipitate in the veins as sometimes valuable ore minerals. The association of gold and quartz veins is a well-known example. Quartz is the most common "gangue mineral" in ore deposits.
Quartz Crystals
Quartz crystals typically grow in fluids at elevated temperatures between 150°C and 600°C, but they also grow at ambient conditions (Mackenzie and Gees, 1971; Ries and Menckhoff, 2008).
Quartz is best known for the beautiful crystals it forms in all sorts of cavities and fissures. The greatest variety of shapes and colors of quartz crystals comes from hydrothermal ore veins and deposits, reflecting large differences in growth conditions in these environments (chemistry, temperature, pressure). Splendid, large crystals grow from ascending hot brines in large fissures, from residual silica-rich fluids in cavities in pegmatites and from locally mobilized silica in Alpine-type fissures. An economically important source of amethyst for the lapidary industry are cavities of volcanic rocks. Small, but well-formed quartz crystals are found in septarian nodules, and in dissolution pockets in limestones.
Well-formed quartz crystals that are fully embedded in sedimentary rocks and grew during diagenesis (so-called authigenic quartz crystals) are occasionally found in limestones, marls, and evaporites (e.g. Rykart, 1984).
Euhedral quartz crystals that are embedded in igneous rocks are uncommon. Quartz is among the last minerals that form during the solidification of a magma, and because the crystals fill the residual space between the older crystals of other minerals they are usually irregular. Euhedral, stubby bipyramidal quartz crystals are occasionally found in rhyolites. These are usually paramorphs after beta-quartz with hexagonal symmetry, quartz crystals whose trigonal habit shows that they grew as alpha-quartz are very rare in volcanic rocks (e.g. Flick and Weissenbach, 1978). Only rarely are euhedral quartz crystals seen embedded in metamorphic rocks (Kenngott, 1854; Tschermak, 1874; Heddle, 1901).
Identification
In most cases quartz is easy to identify by its combination of the following properties:
- hardness (easily scratches glass, also harder than steel)
- glass-like luster
- poor to indistinct cleavage
- conchoidal fracture in crystals, in massive specimens the fracture often looks irregular to the naked eye, but still conchoidal at high magnification.
Note that in macrocrystalline quartz the fracture surfaces have a vitreous to resinous luster, whereas in cryptocrystalline quartz (chalcedony) fractured surfaces are dull.
Crystals are very common and their usually six-sided shape and six-sided pyramidal tips are well-known. Intergrown crystals without tips can often be recognized by the presence of the characteristic striation on the prism faces.
Quartz as a rock-forming mineral, in particular as irregular grains in the matrix, occasionally poses problems and may require additional means of identification. It may be confused with cordierite (pleochroic, tendency to alteration) and nepheline (lower hardness, geological environment incompatible with quartz).
In thin sections macrocrystalline quartz appears clear and homogeneous, with blue-gray to white or bright yellow interference colors and a low relief. Quartz does not show alterations at grain boundaries. Strained quartz grains from metamorphic rocks show a so-called "undulatory extinction" (Blatt and Christie, 1963).
ID Requirements on Mindat
Quartz is one of the few minerals on Mindat where "visual identification" may be accepted as a method of identification for new locality entries and photos of well-formed crystals. In other cases, at least hardness should be checked, too.
For quartz as a rock-forming mineral visual identification is often insufficient.
Handling Quartz
Quartz normally does not require special attention when handled or stored. At ambient conditions, quartz is chemically almost inert, so it does not suffer from the problems seen in many other minerals. Crystals do not disintegrate or crumble, they do not oxidize or dissolve easily in water and they don't mind being touched. The only problem for the collector is dust, which will find and cover your crystals, no matter what you do.
Quartz crystals that contain large fluid or gas inclusions may crack when heated - skeleton quartz is the most sensitive variety in this respect - but most quartz specimens can take some heat, like cleaning in warm water, without being damaged.
Quartz is hard but quite brittle, and with some effort, one can damage a crystal even with things that are much softer. The edges of the crystals are very often slightly damaged because crystals were not kept separate from each other.
Colored quartz varieties can pale in sunlight, the most sensitive variety is euhedral rose quartz/pink quartz, which should be kept in the dark. Amethyst, smoky quartz and natural citrine will also pale, but it takes very long.
Mild ultrasonic cleaning is usually not a problem as long the crystals are not internally cracked, but some varieties may be damaged, in particular, amethyst (due to its polysynthetic Brazil law twinning) and skeleton quartz with liquid and gas inclusions.
Rock Currier wrote a Mindat article on cleaning quartz that is worthwhile reading: http://www.mindat.org/article.php/403/Cleaning+Quartz
When cutting, grinding and polishing specimens, keep in mind that quartz dust will cause silicosis (for a review, see Goldsmith, 1994), do not cut or grind dry and wear an appropriate dust mask.
Quartz bear, on average, 10 ppmw (5 ppmw median) of water. Crystals rich in OH defects may bear as much as 250 ppmw (maximum).
 Visit gemdat.org for gemological information about Quartz.
Visit gemdat.org for gemological information about Quartz.Unique Identifiers
Mindat ID:
3337
Long-form identifier:
mindat:1:1:3337:0
GUID
(UUID V4):
(UUID V4):
4ca61d6f-75f8-4208-8fb2-3b0eecbcd8f0
IMA Classification of Quartz
Approved, 'Grandfathered' (first described prior to 1959)
Classification of Quartz
4.DA.05
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
D : Metal: Oxygen = 1:2 and similar
A : With small cations: Silica family
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
D : Metal: Oxygen = 1:2 and similar
A : With small cations: Silica family
Dana 7th ed.:
75.1.3.1
75.1.3.1
75 : TECTOSILICATES Si Tetrahedral Frameworks
1 : Si Tetrahedral Frameworks - SiO2 with [4] coordinated Si
75 : TECTOSILICATES Si Tetrahedral Frameworks
1 : Si Tetrahedral Frameworks - SiO2 with [4] coordinated Si
7.8.1
7 : Oxides and Hydroxides
8 : Oxides of Si
7 : Oxides and Hydroxides
8 : Oxides of Si
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Qz | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Qtz | Kretz (1983) | Kretz, R. (1983) Symbols of rock-forming minerals. American Mineralogist, 68, 277–279. |
| Qtz | Siivolam & Schmid (2007) | Siivolam, J. and Schmid, R. (2007) Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: List of mineral abbreviations. Web-version 01.02.07. IUGS Commission on the Systematics in Petrology. download |
| Qz | Whitney & Evans (2010) | Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 doi:10.2138/am.2010.3371 |
| Qtz | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
| Qz | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Pronunciation of Quartz
Pronunciation:
| Play | Recorded by | Country |
|---|---|---|
| Jolyon Ralph | United Kingdom |
Physical Properties of Quartz
Vitreous
Transparency:
Transparent, Translucent
Colour:
Colorless, purple, rose, red, black, yellow, brown, green, blue, orange, etc.
Streak:
White
Hardness:
7 on Mohs scale
Hardness Data:
Mohs hardness reference species
Comment:
Some variability by direction.
Tenacity:
Brittle
Cleavage:
Poor/Indistinct
The rhombohedral cleavage r{1011} is most often seen, there are at least six others reported.
The rhombohedral cleavage r{1011} is most often seen, there are at least six others reported.
Fracture:
Conchoidal
Comment:
Tough when massive
Density:
2.65 - 2.66 g/cm3 (Measured) 2.66 g/cm3 (Calculated)
Optical Data of Quartz
Type:
Uniaxial (+)
RI values:
nω = 1.544(1) nε = 1.553(1)
Max Birefringence:
δ = 0.009

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Low
Dispersion:
low
Comments:
Varieties colored by trace elements built into the crystal lattice, as opposed to varieties colored by inclusions, generally show dichroism: smoky quartz, amethyst, citrine, prasiolite, "rose quartz in crystals" (a.k.a. pink quartz), are pleochroic.
Chemistry of Quartz
Mindat Formula:
SiO2
Elements listed:
Common Impurities:
H,Al,Li,Fe,Ti,Na,Mg,Ge,etc
Age distribution
Recorded ages:
Phanerozoic : 279 ± 3 Ma to 55.7 Ma - based on 7 recorded ages.
Crystallography of Quartz
Crystal System:
Trigonal
Class (H-M):
3 2 - Trapezohedral
Space Group:
P31 2 1
Cell Parameters:
a = 4.9133 Å, c = 5.4053 Å
Ratio:
a:c = 1 : 1.1
Unit Cell V:
113.00 ų (Calculated from Unit Cell)
Z:
3
Twinning:
Dauphiné law.
Brazil law.
Japan law.
Others for beta-quartz...
Brazil law.
Japan law.
Others for beta-quartz...
Comment:
Space group is P3121 for left-handed crystals and P3221 for right-handed crystals
Crystallographic forms of Quartz
Crystal Atlas:
Image Loading
Click on an icon to view
3d models and HTML5 code kindly provided by
www.smorf.nl.
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0000789 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 0 | 293 | |
| 0000790 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 2.07 | 293 | |
| 0000791 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 3.76 | 293 | |
| 0000792 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 4.86 | 293 | |
| 0000793 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 5.58 | 293 | |
| 0000794 | Quartz | Levien L, Prewitt C T, Weidner D J (1980) Structure and elastic properties of quartz at pressure American Mineralogist 65 920-930 |  | 1980 | 6.14 | 293 | |
| 0004265 | Quartz | Ikuta D, Kawame N, Banno S, Hirajima T, Ito K, Rakovan J F, Downs R T, Tamada O (2007) First in situ X-ray diffraction identification of coesite and retrograde quartz on a glass thin section of an ultrahigh-pressure metamorphic rock and their crystal structure details American Mineralogist 92 57-63 |  | 2007 | Yangkou meta-igneous complex in the middle part of the Sulu UHP terrain, eastern China | 0 | 293 |
| 0004266 | Quartz | Ikuta D, Kawame N, Banno S, Hirajima T, Ito K, Rakovan J F, Downs R T, Tamada O (2007) First in situ X-ray diffraction identification of coesite and retrograde quartz on a glass thin section of an ultrahigh-pressure metamorphic rock and their crystal structure details American Mineralogist 92 57-63 |  | 2007 | Oomine granite, Tenkawa-mura, Nara, Southwest Japan | 0 | 293 |
| 0004267 | Quartz | Ikuta D, Kawame N, Banno S, Hirajima T, Ito K, Rakovan J F, Downs R T, Tamada O (2007) First in situ X-ray diffraction identification of coesite and retrograde quartz on a glass thin section of an ultrahigh-pressure metamorphic rock and their crystal structure details American Mineralogist 92 57-63 |  | 2007 | Oomine granite, Tenkawa-mura, Nara, Southwest Japan | 0 | 293 |
| 0006212 | Quartz | Antao S M, Hassan I, Wang J, Lee P L, Toby B H (2008) State-of-the-art high-resolution powder x-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite The Canadian Mineralogist 46 1501-1509 |  | 2008 | not specified | 0 | 293 |
| 0006362 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 298 | ||
| 0006363 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 398 | ||
| 0006364 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 498 | ||
| 0006365 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 597 | ||
| 0006366 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 697 | ||
| 0006367 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 773 | ||
| 0006368 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 813 | ||
| 0006369 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 838 | ||
| 0006370 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 848 | ||
| 0006371 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 854 | ||
| 0006372 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 859 | ||
| 0006373 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 869 | ||
| 0006374 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 891 | ||
| 0006375 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 920 | ||
| 0006376 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 972 | ||
| 0006377 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 1012 | ||
| 0006378 | Quartz | Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure European Journal of Mineralogy 2 63-77 | 1990 | 0 | 1078 | ||
| 0008971 | Quartz | Rosa A L, El-Barbary A A, Heggie M I, Briddon P R (2005) Structural and thermodynamic properties of water related defects in alpha-quartz Physics and Chemistry of Minerals 32 323-331 | 2005 | 0 | 293 | ||
| 0018071 | Quartz | Wyckoff R (1926) Kriterien fur hexagonale Raumgruppen und die Kristallstruktur von beta Quarz. _cod_database_code 1011200 Zeitschrift fur Kristallographie 63 507-537 | 1926 | 0 | 293 | ||
| 0017992 | Quartz | Wei (1935) Die Bindung im Quarz _cod_database_code 1011097 Zeitschrift fur Kristallographie 92 355-362 | 1935 | 0 | 293 | ||
| 0010604 | Quartz | Arnold H (1962) Die struktur des hochquarzes Zeitschrift fur Kristallographie 117 467-469 |  | 1962 | 0 | 293 | |
| 0010605 | Quartz | Arnold H (1962) Die struktur des hochquarzes Zeitschrift fur Kristallographie 117 467-469 |  | 1962 | 0 | 293 | |
| 0011007 | Quartz | Glinnemann J, King H E, Schulz H, Hahn T, La Placa S J, Dacol F (1992) Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure Zeitschrift fur Kristallographie 198 177-212 |  | 1992 | 0 | 293 | |
| 0011008 | Quartz | Glinnemann J, King H E, Schulz H, Hahn T, La Placa S J, Dacol F (1992) Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure Zeitschrift fur Kristallographie 198 177-212 |  | 1992 | 4 | 293 | |
| 0011009 | Quartz | Glinnemann J, King H E, Schulz H, Hahn T, La Placa S J, Dacol F (1992) Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure Zeitschrift fur Kristallographie 198 177-212 |  | 1992 | 7.2 | 293 | |
| 0011010 | Quartz | Glinnemann J, King H E, Schulz H, Hahn T, La Placa S J, Dacol F (1992) Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure Zeitschrift fur Kristallographie 198 177-212 |  | 1992 | 10.2 | 293 | |
| 0012866 | Quartz | Gualtieri A F (2000) Accuracy of XRPD QPA using the combined Rietveld-RIR method Journal of Applied Crystallography 33 267-278 | 2000 | Baveno, Novara, Italy | 0 | 293 | |
| 0018749 | Quartz | Gibbs G V, Boisen M B, Downs R T, Lasaga A C (1988) Mathematical Modeling of the structures and bulk moduli of TX2 quartz Materials Research Society Symposia Proceedings 121 155-165 | 1988 | theoretical | 0 | 293 | |
| 0018049 | Quartz | Brill R, Hermann C, Peters C (1939) Studien ueber chemische Bindung mittels Fourieranalyse III. Die Bindung im Quarz _cod_database_code 1011172 Naturwissenschaften 27 676-677 | 1939 | 0 | 293 | ||
| 0015462 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 0 | 293 | |
| 0015463 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 2 | 293 | |
| 0015464 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 5.1 | 293 | |
| 0015465 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 8 | 293 | |
| 0015466 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 9.5 | 293 | |
| 0015467 | Quartz | Hazen R M, Finger L W, Hemley R J, Mao H K (1989) High-pressure crystal chemistry and amorphization of alpha-quartz Solid State Communications 72 507-511 | 1989 | synthetic | 12.5 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Image Loading
Radiation - Copper Kα
Data courtesy of RRUFF project at University of Arizona, used with permission.
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 4.257 Å | (22) |
| 3.342 Å | (100) |
| 2.457 Å | (8) |
| 2.282 Å | (8) |
| 1.8179 Å | (14) |
| 1.5418 Å | (9) |
| 1.3718 Å | (8) |
Geological Environment
Paragenetic Mode(s):
Geological Setting:
Most of them...
Synonyms of Quartz
Other Language Names for Quartz
Arabic:مرو
Bosnian:Kvarc
Bulgarian:Кварц
Catalan:Quars
Croatian:Kvarc
Czech:Křemen
Danish:Kvarts
Dutch:Kwarts
Esperanto:Kvarco
Estonian:Kvarts
Farsi/Persian:کوارتز
Finnish:Kvartsi
French:Quartz
Galician:Cuarzo
Greek:Χαλαζίας
Hebrew:קוורץ
Hungarian:Kvarc
Indonesian:Kuarsa
Irish Gaelic:Grian Cloch
Italian:Quarzo
Korean:석영
Latvian:Kvarcs
Lithuanian:Kvarcas
Luxembourgish:Quarz
Macedonian:Кварц
Malagasy:Vatokaranana
Vatovelona
Vatovelona
Malay:Kuarza
Norwegian:Kvarts
Polish:Kwarc
Portuguese:Quartzo
Romanian:Cuarţ
Russian:Кварц
Serbian:Кварц
Slovak:Kremeň
Slovenian:Kamena strela
Spanish:Cuarzo
Swedish:Kvarts
Thai:ควอตซ์
Traditional Chinese:石英
Turkish:Kuvars
Ukrainian:Кварц
Vietnamese:Thạch anh
Varieties of Quartz
| "Herkimer-style" Quartz | This is a collective name to group together the many different local names for transparent, lustrous quartz crystals, usually doubly-terminated, often associated with inclusions of petroleum and/or associated with oil or coal deposits within sedimentary r... |
| Agate | A distinctly banded fibrous chalcedony. Originally reported from Dirillo river (Achates river), Acate, Ragusa Province, Sicily, Italy. The banding in agate is based on periodic changes in the translucency of the agate substance. Layers appear darker when... |
| Agate-Jasper | A variety of agate consisting of Jasper veined with Chalcedony. |
| Agatized coral | A variety of agate/chalcedony replacing coral. |
| Amarillo Stone | A figured variety of chalcedony. May be the same as Alibates flint. |
| Amberine | Yellow to yellow-green chalcedony variety found in Death Valley, Inyo Co., California, USA. |
| Amethyst | A violet to purple variety of quartz that owes its color to gamma irradiation (Berthelot, 1906) and the presence of traces of iron built into its crystal lattice (Holden, 1925). The irradiation causes the iron Fe(+3) atoms that replace Si in the lattice t... |
| Ametrine | Ametrine crystals are made of alternating sectors of purple and yellow to orange color. Slabs cut perpendicular to the c axis of the crystal look a bit like a pinwheel. The purple sectors are situated under the positive rhombohedral faces (r), and the yel... |
| Apricotine | Reddish-yellow waterworn apricot-coloured quartz pebbles. Originally described from Sunset Beach, Cape May, Lower Township, Cape May Co., New Jersey, USA. |
| Aquaprase | Aquaprase is a registered trademark of Melas, Ionannis Bloumstrom and Chordia, Avant Kumar who are marketing this material. A bluish green chalcedony, colored by chromium and nickel, is marketed under the trade name “Aquaprase.” Origin is an unspecif... |
| Arkansas Candle | A cluster of clear Quartz crystals in a candle-like formation. Also single crystals that show a greater than 7 to 1 length to width ratio. |
| Aventurine | A variety of quartz containing glistening fragments (usually mica, such as fuchsite, but also hematite), which can be cut and polished as a gemstone. Most commonly when the general public encounter this stone it is in the form of green stone beads that ca... |
| Azurchalcedony | Chalcedony coloured by Chrysocolla, from Arizona, USA |
| Babel-Quartz | A historical name given for a variety of quartz named for the fancied resemblance of the crystals to the successive tiers of the Tower of Babel. In some cases, but not all, the morphology is caused by growth inhibition by other minerals (later dissolved... |
| Beekite | A name given to Chalcedony pseudomorphs after coral or shells. Originally described from Devon, England, UK. |
| Binghamite | Binghamite refers to a diverse group of lapidary materials from the mines on the Cuyuna North Iron Range in Crow Wing County, Minnesota. It is related to Minnesota silkstone and Minnesota tigers' eye. In fact all three materials can be found in the same s... |
| Bird's Eye Agate | A variety of eye agate where the eyes are supposed to resemble the eyes of a bird. |
| Blue Chalcedony | Blue colour caused by the Tyndall effect (light scattering by colloid sized particles). Transmitted light looks yellowish or reddish rather than blue. |
| Blue Lace Agate | A pale blue banded variety of Agate (Chalcedony). |
| Blue Quartz | An opaque to translucent, blue variety of quartz, owing its colour to inclusions, commonly of fibrous magnesioriebeckite or crocidolite, or of tourmaline. The color may be caused by the color of the included minerals or by Rayleigh scattering of light at ... |
| Botswana Agate | A variety of agate from Botswana, banded with fine, parallel lines, often coloured pink blending into white. |
| Brecciated Agate | A naturally cemented matrix of broken agate fragments. |
| Buhrstone | A cellular flinty material used for millstones. |
| Bull Quartz | Milky to greyish, massive. |
| Burnt amethyst | Heated amethyst; the heating results in a yellow-orange, yellow-brown, or dark brownish colour. Often incorrectly sold as citrine. |
| Cactus Quartz | Quartz crystals encrusted by a second generation of smaller crystals grown on the prism faces. The small second generation crystals point away from the prism and their orientation is not related to the crystallographic orientation of the central crystal. ... |
| Cape May Diamond | Waterworn transparent quartz pebbles. A locally applied marketing name/ploy to clear, colorless quartz beach pebbles occurring along the Delaware Bay beaches of Cape May County, New Jersey, USA. Cut stones from these pebbles are sold in tourist areas of t... |
| Capped Quartz | Quartz crystals made of loosely connected or easily separable parts that correspond to different growth phases. This is caused by the deposition of thin continuous layers of, for example, clay minerals, on the crystal during growth. The typical result is ... |
| Carnelian | A reddish variety of Chalcedony. |
| Chalcedony | Depending on the context, the term "chalcedony" has different meanings. 1. A more general term for all varieties of quartz that are made of microscopic or submicroscopic crystals, the so-called microcrystalline varieties of quartz. Examples are the diffe... |
| Chrome-Chalcedony | A variety of chalcedony colored deep green by Cr compounds. (Compare with the more common chrysoprase variety of chalcedony, which is colored by nickel.) Chrome chalcedony found in an ancient Roman gem collection may have come from one of the chromium dep... |
| Citrine | A yellow to yellow-orange or yellow-green variety of quartz. Quartz colored by inclusions, or coatings, of any kind is not called citrine. Iron-stained quartz should not be mistaken for citrine. A yellow-green citrine crystal with smoky phantoms.A cut ... |
| Clear Lake Diamond | Quartz crystals from the Manke Ranch, Lake County, California. |
| Cloud Agate | Greyish agate with patches of blurry, foggy inclusions. |
| Cotterite | A variety of quartz with "metallic pearly lustre" coating normal quartz crystals. Originally described from a carboniferous limestone quarry at Rock Forest, Mallow, Co. Cork, Ireland. |
| Crazy Lace Agate | An agate composed of multicoloured twisting and turning bands. |
| Cubosilicite | Pseudomorphs of Chalcedonly after Fluorite - small blue cubes |
| Damsonite | Trade name for a light violet to dark purple chalcedony from Arizona. |
| Dendritic Agate | Chalcedony containing dendritic inclusions. |
| Diackethyst | A local name for translucent wine and amethystine coloured chalcedony pebbles. Originally described from Craig, Montrose, Tayside (Angus), Scotland, UK. |
| Dotsero Diamond | Fanciful local name for quartz crystals enclosed in a geologically recent basalt flow. Being incompatible with basaltic lava, the quartz crystals are rounded by reaction with the surrounding lava. Apparently the crystals were detrital, and got picked up b... |
| Dragonite | A rounded quartz pebble representing a quartz crystal that has lost its brilliancy and angular form; in gravels, once believed to be a fabulous stone obtained from the head of a flying dragon. |
| Eisenkiesel | A quartz that is coloured red, orange, or brown by hematite inclusions. Translucent to almost opaque. The term "eisenkiesel" is sometimes also used in a wider sense, as a synonym of ferruginous quartz, for any quartz with iron oxides and hydroxide minera... |
| El Doradoite | Trade name for blue quartz or chalcedony. Originally described from El Dorado Co., California, USA. |
| Ema egg | Trade name for a river-tumbled pebble of transparent quartz with a frosted exterior resembling an egg shell, originally collected from rivers in Brazil, with one side sawn flat and polished as a window to view the interior. Pebbles of quartz and other min... |
| Enhydro Agate | An agate nodule partly filled with water. |
| Eye Agate | Agate with concentric ring pattern, looking like an eye. |
| Faden Quartz | "Faden quartz" is the anglicized version of the German "Fadenquarz". "Faden" (pronounced "fah-den") means "thread" and refers to a white line that runs through the crystal. In French, these are called " quartz a âme " Faden quartz forms in fissures in t... |
| Fairburn Agate | A unique and rare variety of Fortification Agate from Fairburn, Custer Co., South Dakota, USA. |
| Fensterquarz | Literally "window quartz". Skeletal quartz which has rhombohedral faces appearing like windows. |
| Ferruginous Quartz | A variety of quartz colored red, brown, or yellow by inclusions of hematite or limonite, and usually massive and opaque. |
| Fire Agate | A variety of chalcedony containing inclusions of goethite or limonite, producing an iridescent effect or "fire." |
| Fortification Agate | Agate with sharp-angled bands which resemble the outlines of fortifications of a castle. |
| Fossil Agate | Agate as a replacement material in fossils. |
| Grape agate | A marketing name for aggregates of spheroidal amethystine quartz/chalcedony most notably applied to material from Indonesia. Individual spheres measure 2 to 20 millimeters in diameter and consist of thin radially grown "fibers" made of quartz. In spit... |
| Haema-ovoid-agates | Name proposed for a reddish agate with ovoidal patches of cacholong, etc. |
| Hair Amethyst | A name for acicular crystals of Amethyst. |
| Haytorite | Although the original specimens from Haytor Mine were pseudomorphs of quartz after datolite, the name has been frequently used in Cornwall also for quartz pseudomorphs after a veriety of other minerals, including calcite dolomite and siderite (see e.g. Co... |
| Herbeckite | A variety of Agate or Jasper impregnated with Iron Hydrate. [Clark, 1993 - "Hey's Mineral Index"] Originally described from Hrbek Mine, Svatá Dobrotivá (St Benigna), Beroun (Beraun), Central Bohemia Region, Bohemia (Böhmen; Boehmen), Czech Republic. |
| Iris Agate | An iridescent variety of agate - when sliced into a thin section it exhibits all the colours of the spectrum when viewed in transmitted light. |
| Iris Quartz | Quartz crystals displaying internal spectral colours under minor rhombohedral faces. This interference phenomenon is due to reflection and refraction on extremely thin parallel Brazil-law twinning lamellae or periodic etching of defects on z faces, result... |
| Jacinto de Compostela | In Spanish mineralogical literature, the name is traditionally used exclusively for the red "floater" variety of authigenic quartzes from continental gypsum-bearing marls of the Triassic Keuper formation. (They may also be found occasionally in younger Te... |
| Keystonite Chalcedony | A local trade name for Chalcedony coloured blue by Chrysocolla. |
| Laguna Agate | A colourful agate variety. Originally described from Ojo Laguna, Chihuahua, Mexico. |
| Lake Superior Agate | Believed to be the world's oldest agates, over 1 billion years old, these are found throughout the northern US having been spread from the original Lake Superior region by glaciation. It has generally pale colouring. |
| Landscape Agate | A variety of chalcedony with inclusions giving the appearance of a landscape scene. |
| Lithium Quartz | A name in common trade use for a pink/purple translucent to opaque variety of quartz, possibly containing inclusions of a lithium-rich mineral such as lepidolite - however it could equally be a misleading/incorrect name, and should be regarded a simply a ... |
| Macromosaic Quartz | Prism surface of a macromosaic quartzNormal habit quartz crystal with macromosaic structure from an alpine-type fissure Quartz crystals that are composed of slightly tilted and radially arranged wedge-shaped sectors. They can be recognized by the presence... |
| Mexican Lace Agate | Lacy or wavy agate from Mexico. |
| Milky Quartz | A semi-transparent to opaque white-coloured variety of quartz. |
| Mocha Stone | A variety of agate (chalcedony) containing inclusions of pyrolusite. Originally described from Mocha, Saudi Arabia. |
| Moqi agate | |
| Moss Agate | A variety of Chalcedony frequently containing green mineral inclusions (eg Chlorite, Hornblende, etc.) or brown to black dendrites of iron or manganese oxides. |
| Mutzschen Diamonds | Clear variety of quartz (rock crystal) from Mutzschen, Saxony. Occurs in voids of Permian volcanic rocks (rhyolites). |
| Myrickite | Local name for a chalcedony with grey ground and red spots (inclusions of cinnabar). Originally described from Myrick Spring, San Bernardino Co., California, USA. |
| Nipomo Agate | Chalcedony with inclusions of Marcasite. Originally described from Nipomo, San Luis Obispo Co., California, USA. |
| Oil Quartz | A variety of Quartz from Tyrol, Austria, which contains yellow stains in cracks. BM 1924,110 and 111 are two specimens in the Natural History Museum, London. [Clark, 1993 - "Hey's Mineral Index"] |
| Onyx | In correct usage, the name refers to a (usually) black and white banded variety of agate, or sometimes a monochromatic agate with dark and light parallel bands (brown and white for example) - but traditionally the name was reserved for black and white ban... |
| Pecos Diamonds | Colourful, doubly-terminated quartz crystals that occur in the Permian Seven Rivers Formation along the Pecos River valley in southeastern New Mexico. |
| Phantomquarz | A variety of quartz that shows one or more phantoms. (See phantom crystal). |
| Pietersite | Chalcedony with embedded fibers of amphibole minerals with varying degrees of alteration. Blue-gray, brown and yellow colors. The fibers cause a chatoyancy similar to that seen in tiger's eye, but tiger's eye is not made of chalcedony, it is macrocrystall... |
| Pigeon Blood Agate | A blood-red and white variety of agate from Utah. |
| Plasma | A microgranular or microfibrous form of chalcedony coloured in various shades of green by disseminated silicate particles (variously attributed to celadonite, chlorite, amphibole, etc.). Various descriptions of plasma include "of a dullish green color w... |
| Plume Agate | A variety of chalcedony with contrasting colored, plume-like structures within the material. Compare with moss agate. |
| Prase | Originally, the varietal name "prase" was applied to a dull leek-green colored quartzite (a rock, not a mineral*); but over the years it has been also applied to other materials, particularly a green colored jasper of similar color. For perhaps more than... |
| Prase-malachite | A term for Prase enclosing Malachite. |
| Prasiolite | A green transparent variety of macrocrystalline quartz. Compare with prase and plasma. Not to be confused with prasolite! |
| Pseudocubic Quartz | Crystals with a (pseudo)cubic appearance that are dominated by a single rhombohedral form (usually r, { 1 0 -1 1 }). Since the angles of the rhombohedron differ only very little from that of a perfect cube (85.2° and 94.8°, respectively, instead of 90°... |
| Quartz Gwindel | Quartz crystals that grew along and are slightly rotated around a single a-axis. This results in twisted and tabular crystals. The twist reflects the handedness of the quartz crystals. With increasing distance from the base - right-handed gwindels twist c... |
| Quartzine | Quartzine is a fibrous variety of chalcedony. It is also called "length-slow chalcedony" and is usually intergrown with another, more common type of fibrous chalcedony, "length-fast chalcedony", that comprises most of the different varieties of chalcedony... |
| Riband Agate | According to Hey's 3rd Ed. this is 'a banded agate', which doesn't tell us much! |
| Rock Crystal | A transparent colourless variety of quartz. |
| Rose Quartz | Two varieties of quartz are commonly called "rose quartz". 1. One is found in translucent masses made of intergrown anhedral crystals. It occurs in different hues of pink, sometimes bluish, sometimes more reddish; irradiation may cause the formation of ... |
| Rutilated Quartz | Quartz shot through with needles of Rutile. |
| Sagenite (of Kunz) | A redefinition by Kunz in 1892 (possibly a misunderstanding) of the original name Sagenite as defined by Saussure to refer to a variety of quartz - see also Sagenite (of Saussure) and Rutilated Quartz - a more common modern name to refer to Quartz contain... |
| Sard | A brown to brownish-red translucent variety of chalcedony. Pliny the Elder stated that it was named after Sardis, in Lydia, where it was first discovered; but the name probably came with the stone from Persia (Persian sered = yellowish-red). |
| Sardonyx | A variety of Agate with reddish-brown and either black or white bands. |
| Sceptre Quartz | Sceptre quartzes (American English spelling: Scepter quartzes) are crystals in which a second generation crystal tip grew on top of another quartz crystal. In a typical scepter quartz, the younger tip is larger than the first tip, but it may also be small... |
| Schwimmstein | Earthy quartz, as nodular to mamillary masses, as coating on flint. Specific weight < 1, therefore floating on water. |
| Seftonite | A translucent, moss green variety of chalcedony. |
| Shocked Quartz | Quartz shocked under intense pressure (but limited temperature). During the pressure shock, the crystalline structure of quartz will be deformed along planes inside the crystal. These planes, which show up as lines under a microscope, are called planar de... |
| Smoky Quartz | A smoky-gray, brown to black variety of quartz that owes its color to gamma irradiation and the presence of traces of aluminum built into its crystal lattice (Griffiths et al, 1954; O'Brien, 1955). The irradiation causes the aluminum Al(+3) atoms that rep... |
| Snakeskin Agate | Chalcedony with snakeskin-like surface pattern. |
| Star Quartz | Refers to the shape of an aggregate of radiating crystals; not to be confused with the optical property "asterism". Star quartz usually grows at low temperature, often around a core of chalcedony. |
| Suttroper Quarz | Name used for biterminated, milky quartz crystals originally described from Suttrop, Warstein, Sauerland, North Rhine-Westphalia, Germany. Generally used in the plural form, 'Suttroper Quarze', or more correctly (because Suttrop is not the only locality),... |
| Trapiche Quartz | Quartz crystals with a central hexagonal trapiche-like pattern around the c-axis. The trapiche-like pattern is made of voids left around a dendritic seed crystal that forms the core of a normal habit quartz crystal. |
| Youngite | Local name for agate or jasper coated by druzy quartz crystals. Found near Guernsey, Platte Co., Wyoming, USA, in limestone rocks. |
Common Associates
Associated Minerals Based on Photo Data:
| 13,752 photos of Quartz associated with Fluorite | CaF2 |
| 12,426 photos of Quartz associated with Calcite | CaCO3 |
| 10,033 photos of Quartz associated with Pyrite | FeS2 |
| 7,600 photos of Quartz associated with Sphalerite | ZnS |
| 5,641 photos of Quartz associated with Chalcopyrite | CuFeS2 |
| 5,290 photos of Quartz associated with Galena | PbS |
| 4,862 photos of Quartz associated with Hematite | Fe2O3 |
| 4,659 photos of Quartz associated with Siderite | FeCO3 |
| 4,304 photos of Quartz associated with Dolomite | CaMg(CO3)2 |
| 3,542 photos of Quartz associated with Rhodochrosite | MnCO3 |
Related Minerals - Strunz-mindat Grouping
| 4.DA. | Chibaite | SiO2 · n(CH4, C2H6, C3H8, i-C4H10) (n = 3/17 (max)) |
| 4.DA. | Carbon Dioxide Ice | CO2 |
| 4.DA. | Bosoite | SiO2 · nCxH2x+2 |
| 4.DA.10 | Opal | SiO2 · nH2O |
| 4.DA.10 | Tridymite | SiO2 |
| 4.DA.15 | Cristobalite | SiO2 |
| 4.DA.20 | Mogánite | SiO2 |
| 4.DA.25 | Melanophlogite | 46SiO2 · 6(N2,CO2) · 2(CH4,N2) |
| 4.DA.30 | Lechatelierite | SiO2 |
| 4.DA.35 | Coesite | SiO2 |
| 4.DA.40 | Stishovite | SiO2 |
| 4.DA.45 | Keatite | SiO2 |
| 4.DA.50 | Seifertite | SiO2 |
| 4.DA.55 | Quartz-beta | SiO2 |
Other Information
Electrical:
piezoelectric, pyroelectric, may be triboluminescent.
Thermal Behaviour:
Transforms to beta-quartz at 573° C and 1 bar (100 kPa) pressure.
Health Risks:
Quartz is usually quite harmless unless broken or powdered. Broken crystals and masses may have razor-sharp edges that can easily cut skin and flesh. Handle with care. Do not grind dry since long-term exposure to finely ground powder may lead to silicosis.
Industrial Uses:
Ore for silicon, glassmaking, frequency standards, optical instruments, silica source for concrete setting, filtering agents as sand. A major component of sand.
Quartz in petrology
An essential component of rock names highlighted in red, an accessory component in rock names highlighted in green.
- Igneous rock
- Normal crystalline igneous rock
- Exotic crystalline igneous rock
- Pegmatite
- Sedimentary rock and sediment
- Metamorphic rock
- Pyrometamorphic rock
- Queluzite
- Meta-igneous rock
- Metasedimentary rock
- High-grade metamorphic rock
- Very low to low-grade metamorphic rock
- Gneiss
- Schist
- Contact metamorphic rock
- Metasomatic-rock
- Epidosite
- Unclassified rock
Internet Links for Quartz
mindat.org URL:
https://www.mindat.org/min-3337.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Quartz
Reference List:
Adams, Sidney F. (1920) A microscopic study of vein quartz. Economic Geology, 15 (8) 623-664 doi:10.2113/gsecongeo.15.8.623
Tarr, W. A., Lonsdale, John T. (1929) Pseudo-cubic quartz crystals from Artesia, New Mexico. American Mineralogist, 14 (2) 50-53
Drugman, Julien (1939) Prismatic cleavage and steep rhombohedral form in α-quartz. Mineralogical Magazine and Journal of the Mineralogical Society, 25 (164) 259-263 doi:10.1180/minmag.1939.025.164.04
Raman, C. V., Nedungadi, T. M. K. (1940) The α-β Transformation of Quartz. Nature, 145 (3665) 147 doi:10.1038/145147a0
Tomkeieff, S. I. (1942) On the origin of the name ‘quartz’. Mineralogical Magazine and Journal of the Mineralogical Society, 26 (176) 172-178 doi:10.1180/minmag.1942.026.176.04
Frondel, Clifford (1945) History of the quartz oscillator-plate industry, 1941-1944. American Mineralogist, 30 (5-6) 205-213
Frondel, Clifford (1945) Secondary Dauphiné twinning in quartz. American Mineralogist, 30 (5-6) 447-460
Thomas, L. A. (1945) Terminology of Interpenetrating Twins in α-Quartz. Nature, 155 (3936) 424 doi:10.1038/155424a0
Armstrong, Elizabeth (1946) Relation between secondary Dauphiné twinning and irradiation-coloring in quartz. American Mineralogist, 31 (9-10) 456-461
Baker, George (1946) Microscopic quartz crystals in brown coal, Victoria. American Mineralogist, 31 (1-2) 22-30
Faust, George T. (1948) Thermal analysis of quartz and its use in calibration in thermal analysis studies. American Mineralogist, 33 (5-6) 337-345
Gault, H. R. (1949) The frequency of twin types in quartz crystals. American Mineralogist, 34 (3-4) 142-162
Tuttle, O. F. (1949) The variable inversion temperature of quartz as a possible geologic thermometer. American Mineralogist, 34 (9-10) 723-730
Chapman, Carleton A. (1950) Quartz veins formed by metamorphic differentiation of aluminous schists. American Mineralogist, 35 (9-10) 693-710
Brown, C. S., Kell, R. C., Thomas, L. A., Wooster, Nora, Wooster, W. A. (1952) The growth and properties of large crystals of synthetic quartz. Mineralogical Magazine and Journal of the Mineralogical Society, 29 (217) 858-874 doi:10.1180/minmag.1952.029.217.03
Kozu, S. (1952) Japanese twins of quartz. American Journal of Science: Bowen Volume Part 1: 281-292.
Van Praagh, G., Willis, B. T. M. (1952) Striations on Prism Faces of Quartz. Nature, 169 (4302) 623-624 doi:10.1038/169623b0
Frederickson, A. F., Cox, Joseph E. (1954) Mechanism of "solution" of quartz in pure water at elevated temperatures and pressures. American Mineralogist, 39 (11-12) 886-900
Borg, Iris (1956) Note on twinning and pseudo-twinning in detrital quartz grains. American Mineralogist, 41 (9-10) 792-796
de Vries, A. (1958) Determination of the Absolute Configuration of α-Quartz. Nature, 181 (4617) 1193 doi:10.1038/1811193a0
Denning, R. M., Conrad, M. A. (1959) Directional grinding hardness of quartz by peripheral grinding. American Mineralogist, 44 (3-4) 423-428
Foster, Robert J. (1960) Origin of embayed quartz crystals in acidic volcanic rocks. American Mineralogist, 45 (7-8) 892-894
Ballman, A. A. (1961) The growth and properties of colored quartz. American Mineralogist, 46 (3-4) 439-446
Brace, W. F., Walsh, J. B. (1962) Some direct measurements of the surface energy of quartz and orthoclase. American Mineralogist, 47 (9-10) 1111-1122
Blatt, Harvey, Christie, John M. (1963) Undulatory Extinction in Quartz of Igneous and Metamorphic Rocks and Its Significance in Provenance Studies of Sedimentary Rocks. Journal of Sedimentary Research, 33 (3) 559-579 doi:10.1306/74d70ebb-2b21-11d7-8648000102c1865d
Bloss, F. Donald, Gibbs, Gerald V. (1963) Cleavage in quartz. American Mineralogist, 48 (7-8) 821-838
Lang, A. R. (1965) Mapping Dauphiné and Brazil twins in quartz by X-ray topography. Applied Physics Letters, 7 (6) 168-170 doi:10.1063/1.1754361
Dennen, W. H. (1966) Stoichiometric substitution in natural quartz. Geochimica et Cosmochimica Acta, 30 (12) 1235-1241 doi:10.1016/0016-7037(66)90122-0
Lehmann, G., Moore, W. J. (1966) Color Center in Amethyst Quartz. Science, 152 (3725). 1061-1062 doi:10.1126/science.152.3725.1061
McLaren, A. C., Retchford, J. A., Griggs, D. T., Christie, J. M. (1967) Transmission Electron Microscope Study of Brazil Twins and Dislacations Experimentally Produced in Natural Quartz. physica status solidi (b), 19 (2). 631-644 doi:10.1002/pssb.19670190216
Carr, R. M. (1968) The problem of quartz-corundum stability. American Mineralogist, 53 (11-12) 2092-2095
Carstens, H. (1968) A note on the origin of Brazil twins in lamellar quartz. Norsk Geologisk Tidsskrift [Norwegian Journal of Geology], 48 (1-2) 61-64
Carstens, Harald (1968) The lineage structure of quartz crystals. Contributions to Mineralogy and Petrology, 18 (4) 295-304 doi:10.1007/bf00399691
Frondel, Clifford (1968) Quartz twin on. Mineralogical Magazine and Journal of the Mineralogical Society, 36 (282) 861-864 doi:10.1180/minmag.1968.036.282.16
Baumbauer, H. H., Brunner, G. O., Laves, F. (1969) Light scattering of heat-treated quartz in relation to hydrogen-containing defects. American Mineralogist, 54 (5-6) 718-724
McLaren, A. C., Phakey, P. P. (1969) Diffraction Contrast from Dauphiné Twin Boundaries in Quartz. physica status solidi (b), 31 (2). 723-737 doi:10.1002/pssb.19690310233
Carmichael, I. S. E., Nicholls, J., Smith, A. L. (1970) Silica activity in igneous rocks. American Mineralogist, 55 (1-2) 246-263
Feigl, F. J., Anderson, J. H. (1970) Defects in crystalline quartz: Electron paramagnetic resonance of E' vacancy centers associated with germanium impurities. Journal of Physics and Chemistry of Solids, 31. 575-596 doi:10.1016/0022-3697(70)90192-7
Calvert, S. E. (1971) Nature of Silica Phases in Deep Sea Cherts of the North Atlantic. Nature Physical Science, 234. 133-134 doi:10.1038/physci234133a0
Scott, S. D., O'Connor, T. P. (1971) Fluid inclusions in vein quartz, Silverfields Mine, Cobalt, Ontario. The Canadian Mineralogist, 11 (1) 263-271
Bates, John B., Quist, Arvin S. (1972) Polarized Raman Spectra of β‐Quartz. The Journal of Chemical Physics, 56 (4) 1528-1533 doi:10.1063/1.1677402
Baëta, R. D., Ashbee, K. H. G. (1973) Transmission electron microscopy studies of plastically deformed quartz. physica status solidi (a), 18 (1). 155-170 doi:10.1002/pssa.2210180112
Bettermann, Peter, Liebau, Friedrich (1975) The transformation of amorphous silica to crystalline silica under hydrothermal conditions. Contributions to Mineralogy and Petrology, 53 (1) 25-36 doi:10.1007/bf00402452
Donnay, J. D. H., Le Page, Y. (1975) Twin laws versus electrical and optical characters in low quartz. The Canadian Mineralogist, 13 (1) 83-85
Barron, T. H. K., Huang, C. C., Pasternak, A. (1976) Interatomic forces and lattice dynamics of α-quartz. Journal of Physics C: Solid State Physics, 9 (21) 3925-3940 doi:10.1088/0022-3719/9/21/011
Chakraborty, Dipak, Lehmann, Gerhard (1976) Distribution of OH in synthetic and natural quartz crystals. Journal of Solid State Chemistry, 17. 305-311 doi:10.1016/0022-4596(76)90136-5
Chakraborty, D., Lehmann, G. (1976) On the structures and orientations of hydrogen defects in natural and synthetic quartz crystals. physica status solidi (a), 34 (2). 467-474 doi:10.1002/pssa.2210340206
Le Page, Y., Donnay, G. (1976) Refinement of the crystal structure of low-quartz. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, 32 (8) 2456-2459 doi:10.1107/s0567740876007966
Van Goethem, L., Van Landuyt, J., Amelinckx, S. (1977) The α → β transition in amethyst quartz as studied by electron microscopy and diffraction. The interaction of dauphiné with Brazil twins. physica status solidi (a), 41 (1). 129-137 doi:10.1002/pssa.2210410114
Donnay, J. D. H., Le Page, Y. (1978) The vicissitudes of the low-quartz crystal setting or the pitfalls of enantiomorphism. Acta Crystallographica Section A, 34 (4) 584-594 doi:10.1107/s0567739478001242
Robin, Pierre-Yves F. (1979) Theory of metamorphic segregation and related processes. Geochimica et Cosmochimica Acta, 43 (10) 1587-1600 doi:10.1016/0016-7037(79)90179-0
Maschmeyer, D., Niemann, K., Hake, H., Lehmann, G., Räuber, A. (1980) Two modified smoky quartz centers in natural citrine. Physics and Chemistry of Minerals, 6 (2) 145-156 doi:10.1007/bf00311051
Flörke, Otto W., Mielke, Heinz G., Weichert, Jürgen, Kulke, Holger (1981) Quartz with rhombohedral cleavage from Madagascar. American Mineralogist, 66 (5-6) 596-600
Wright, A. F., Lehmann, M. S. (1981) The structure of quartz at 25 and 590°C determined by neutron diffraction. Journal of Solid State Chemistry, 36. 371-380 doi:10.1016/0022-4596(81)90449-7
Bohlen, Steven R., Boettcher, A. L. (1982) The quartz ⇆ coesite transformation: A precise determination and the effects of other components. Journal of Geophysical Research: Solid Earth, 87. 7073-7078 doi:10.1029/jb087ib08p07073
McLaren, A. C., Pitkethly, D. R. (1982) The twinning microstructure and growth of amethyst quartz. Physics and Chemistry of Minerals, 8 (3) 128-135 doi:10.1007/bf00311283
Richet, P., Bottinga, Y., Deniélou, L., Petitet, J.P., Téqui, C. (1982) Thermodynamic properties of quartz, cristobalite and amorphous SiO2: drop calorimetry measurements between 1000 and 1800 K and a review from 0 to 2000 K. Geochimica et Cosmochimica Acta, 46 (12) 2639-2658 doi:10.1016/0016-7037(82)90383-0
Serebrennikov, A. I., Valter, A. A., Mashkovtsev, R. I., Scherbakova, M. Ya. (1982) The investigation of defects in shock-metamorphosed quartz. Physics and Chemistry of Minerals, 8 (4) 153-157 doi:10.1007/bf00308236
Yasuda, Toshikazu, Sunagawa, Ichiro (1982) X-ray topographic study of quartz crystals twinned according to japan twin law. Physics and Chemistry of Minerals, 8 (3) 121-127 doi:10.1007/bf00311282
Maschmeyer, D., Lehmann, G. (1983) A trapped-hole center causing rose coloration of natural quartz. Zeitschrift für Kristallographie - Crystalline Materials, 163 (14) 181 doi:10.1524/zkri.1983.163.14.181
Scandale, E., Stasi, F., Zarka, A. (1983) Growth defects in a quartz druse dislocations. Journal of Applied Crystallography, 16 (4) 399-403 doi:10.1107/s0021889883010687
Sunagawa, I., Yasuda, T. (1983) Apparent re-entrant corner effect upon the morphologies of twinned crystals; A case study of quartz twinned according to Japanese twin law. Journal of Crystal Growth, 65 (1). 43-49 doi:10.1016/0022-0248(83)90034-9
Barker, Colin, Robinson, S. J. (1984) Thermal release of water from natural quartz. American Mineralogist, 69 (11-12) 1078-1081
Weil, John A. (1984) A review of electron spin spectroscopy and its application to the study of paramagnetic defects in crystalline quartz. Physics and Chemistry of Minerals, 10 (4) 149-165 doi:10.1007/bf00311472
Scandale, E., Stasi, F. (1985) Growth defects in quartz druses, pseudo-basal dislocations. Journal of Applied Crystallography, 18 (5) 275-278 doi:10.1107/s0021889885010330
Sawyer, E. W., Robin, P.-Y. F. (1986) The subsolidus segregation of layer-parallel quartz-feldspar veins in greenschist to upper amphibolite facies metasediments. Journal of Metamorphic Geology, 4 (3) 237-260 doi:10.1111/j.1525-1314.1986.tb00350.x
Hemingway, Bruce S. (1987) Quartz: Heat capacities from 340 to 1000 K and revised values for the thermodynamic properties. American Mineralogist, 72 (3-4) 273-279
Hurai, Vratislav, Stresko, Vladimir (1987) Correlation between quartz crystal morphology and composition of fluid inclusions as inferred from fissures in central Slovakia (Czechoslovakia) Chemical Geology, 61 (1) 225-239 doi:10.1016/0009-2541(87)90042-8
Jayaraman, A., Wood, D. L., Maines, R. G. (1987) High-pressure Raman study of the vibrational modes inAlPO4andSiO2(α-quartz) Physical Review B, 35 (16) 8316-8321 doi:10.1103/physrevb.35.8316
Molenaar, N., de Jong, A. F. M. (1987) Authigenic quartz and albite in Devonian limestones: origin and significance. Sedimentology, 34 (4) 623-640 doi:10.1111/j.1365-3091.1987.tb00791.x
Owen, Michael R. (1988) Radiation-damage halos in quartz. Geology, 16 (6) 529 doi:10.1130/0091-7613(1988)016<0529:rdhiq>2.3.co;2
Ramseyer, K., Baumann, J., Matter, A., Mullis, J. (1988) Cathodoluminescence Colours of α-Quartz. Mineralogical Magazine, 52 (368) 669-677 doi:10.1180/minmag.1988.052.368.11
Sowa, H. (1988) The oxygen packings of low-quartz and ReO3 under high pressure. Zeitschrift für Kristallographie - Crystalline Materials, 184 (14) 257-268 doi:10.1524/zkri.1988.184.14.257
Davidson, Paula M., Lindsley, Donald H. (1989) Thermodynamic analysis of pyroxene-olivine-quartz equilibria in the system CaO-MgO-FeO-SiO2. American Mineralogist, 74 (1-2) 18-30
Rao, P. S., Weil, J. A., Williams, J. A. S. (1989) EPR investigation of carbonaceous natural quartz single crystals. The Canadian Mineralogist, 27 (2) 219-224
Blum, Alex E, Yund, Richard A, Lasaga, Antonio C (1990) The effect of dislocation density on the dissolution rate of quartz. Geochimica et Cosmochimica Acta, 54 (2) 283-297 doi:10.1016/0016-7037(90)90318-f
Brady, Patrick V., Walther, John V. (1990) Kinetics of quartz dissolution at low temperatures. Chemical Geology, 82. 253-264 doi:10.1016/0009-2541(90)90084-k
Dove, Patricia M., Crerar, David A. (1990) Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochimica et Cosmochimica Acta, 54 (4) 955-969 doi:10.1016/0016-7037(90)90431-j
Kihara, Kuniaki (1990) An X-ray study of the temperature dependence of the quartz structure. European Journal of Mineralogy, 2 (1) 63-78 doi:10.1127/ejm/2/1/0063
Ribet, I., Thiry, M. (1990) Quartz growth in limestone: Example from water-table silicification in the Paris basin. Chemical Geology, 84 (1) 316-319 doi:10.1016/0009-2541(90)90250-b
Taijing, Lu, Sunagawa, Ichiro (1990) Structure of Brazil twin boundaries in amethyst showing brewster fringes. Physics and Chemistry of Minerals, 17 (3) 207-211 doi:10.1007/bf00201451
Cordier, Patrick, Doukhan, Jean Claude (1991) Water speciation in quartz: A near infrared study. American Mineralogist, 76 (3-4) 361-369
Heaney, Peter J., Veblen, David R. (1991) Observations of the α-β phase transition in quartz: A review of imaging and diffraction studies and some new results. American Mineralogist, 76 (5-6) 1018-1032
Glinnemann, J., King Jr, Η. E., Schulz, H., Hahn, Th., La Placa, S. J., Dacol, F. (1992) Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure. Zeitschrift für Kristallographie - Crystalline Materials, 198 (14) 177 doi:10.1524/zkri.1992.198.14.177
Peucker-Ehrenbrink, B., Behr, H.-J. (1993) Chemistry of hydrothermal quartz in the post-Variscan “Bavarian Pfahl” system, F.R. Germany. Chemical Geology, 103 (1) 85-102 doi:10.1016/0009-2541(93)90293-r
Rink, W.J., Rendell, H., Marseglia, E.A., Luff, B.J., Townsend, P.D. (1993) Thermoluminescence spectra of igneous quartz and hydrothermal vein quartz. Physics and Chemistry of Minerals, 20 (5) 353-361 doi:10.1007/bf00215106
Cordier, Patrick, Weil, John A., Howarth, David F., Doukhan, Jean Claude (1994) Influence of the (4H)Si defect on dislocation motion in crystalline quartz. European Journal of Mineralogy, 6 (1) 17-22 doi:10.1127/ejm/6/1/0017
Swamy, V., Saxena, Surendra K., Sundman, Bo, Zhang, J. (1994) A thermodynamic assessment of silica phase diagram. Journal of Geophysical Research: Solid Earth, 99. 11787-11794 doi:10.1029/93jb02968
Dong, Guoyi, Morrison, Gregg, Jaireth, Subhash (1995) Quartz textures in epithermal veins, Queensland; classification, origin and implication. Economic Geology, 90 (6) 1841-1856 doi:10.2113/gsecongeo.90.6.1841
Onasch, Charles M., Vennemann, Torsten W. (1995) Disequilibrium partitioning of oxygen isotopes associated with sector zoning in quartz. Geology, 23 (12) 1103 doi:10.1130/0091-7613(1995)023<1103:dpooia>2.3.co;2
Kalceff, M. A. Stevens, Phillips, M. R. (1995) Cathodoluminescence microcharacterization of the defect structure of quartz. Physical Review B, 52 (5) 3122-3134 doi:10.1103/physrevb.52.3122
Gratz, A (1996) Distinguishing shocked from tectonically deformed quartz by the use of the SEM and chemical etching. Earth and Planetary Science Letters, 142 (3) 513-521 doi:10.1016/0012-821x(96)00099-4
Carpenter, Michael A., Salje, Ekhard K. H., Graeme-Barber, Ann, Wruck, Bernd, Dove, Martin T., Knight, Kevin S. (1998) Calibration of excess thermodynamic properties and elastic constant variations associated with the alpha <-->beta phase transition in quartz. American Mineralogist, 83 (1) 2-22 doi:10.2138/am-1998-1-201
Gautier, J.-M. (1998) An Experimental Study of Quartz Precipitation and Dissolution Rates at 200°C. Mineralogical Magazine, 62 (1) 509-510 doi:10.1180/minmag.1998.62a.1.270
Bachheimer, Jean-Pierre (2000) Comparative NIR and IR examination of natural, synthetic, and irradiated synthetic quartz. European Journal of Mineralogy, 12 (5) 975-986 doi:10.1127/0935-1221/2000/0012-0975
Ghent, E. D., Stout, M. Z. (2000) Mineral equilibria in quartz leucoamphibolites (quartz—garnet—plagioclase—hornblende calc-silicates) from southeastern British Columbia, Canada. The Canadian Mineralogist, 38 (1) 233-244 doi:10.2113/gscanmin.38.1.233
Götze, J., Plötze, M., Habermann, D. (2001) Origin, spectral characteristics and practical applications of the cathodoluminescence (CL) of quartz - a review. Mineralogy and Petrology, 71 (3) 225-250 doi:10.1007/s007100170040
Skála, R., Hörz, F. (2001) Unit-cell dimensions of experimentally shock-loaded quartz revisited. Meteoritics & Planetary Science, 36. 192-193 doi:10.1111/j.1945-5100.2001.tb01534.x
Schlegel, Michel L., Nagy, Kathryn L., Fenter, Paul, Sturchio, Neil C. (2002) Structures of quartz (100)- and (101)-water interfaces determined by x-ray reflectivity and atomic force microscopy of natural growth surfaces. Geochimica et Cosmochimica Acta, 66 (17) 3037-3054 doi:10.1016/s0016-7037(02)00912-2
Rodgers, K. A., Hampton, W. A. (2003) Laser Raman identification of silica phases comprising microtextural components of sinters. Mineralogical Magazine, 67 (1) 1-13 doi:10.1180/0026461036710079
Wangen, Magnus, Munz, Ingrid Anne (2004) Formation of quartz veins by local dissolution and transport of silica. Chemical Geology, 209 (3) 179-192 doi:10.1016/j.chemgeo.2004.02.011
Basile-Doelsch, Isabelle, Meunier, Jean Dominique, Parron, Claude (2005) Another continental pool in the terrestrial silicon cycle. Nature, 433 (7024). 399-402 doi:10.1038/nature03217
Botis, S., Nokhrin, S. M., Pan, Y., Xu, Y., Bonli, T., Sopuck, V. (2005) Natural radiation-induced damage in quartz. I. Correlations between cathodoluminescence colors and paramagnetic defects. The Canadian Mineralogist, 43 (5) 1565-1580 doi:10.2113/gscanmin.43.5.1565
Götze, Jens, Plötze, Michael, Trautmann, Toralf (2005) Structure and luminescence characteristics of quartz from pegmatites. American Mineralogist, 90 (1) 13-21 doi:10.2138/am.2005.1582
Choudhury, N., Chaplot, S. L. (2006) Ab initio studies of phonon softening and high-pressure phase transitions of α-quartz SiO2. Physical Review B, 73 (9) doi:10.1103/physrevb.73.094304
Grimmer, Hans (2006) Quartz aggregates revisited. Acta Crystallographica Section A Foundations of Crystallography, 62 (2). 103-108 doi:10.1107/s0108767305036950
Enami, M., Nishiyama, T., Mouri, T. (2007) Laser Raman microspectrometry of metamorphic quartz: A simple method for comparison of metamorphic pressures. American Mineralogist, 92 (8) 1303-1315 doi:10.2138/am.2007.2438
Hebert, L. B., Rossman, G. R. (2008) Greenish quartz found at the Thunder Bay Amethyst Mine Panorama, Thunder Bay, Ontario, Canada. The Canadian Mineralogist, 46 (1) 111-124 doi:10.3749/canmin.46.1.111
Botis, S. M., Pan, Yuanming (2009) Theoretical calculations of [AlO4/M+]0 defects in quartz and crystal-chemical controls on the uptake of Al. Mineralogical Magazine, 73 (4) 537-550 doi:10.1180/minmag.2009.073.4.537
Lehmann, K., Berger, A., Götte, T., Ramseyer, K., Wiedenbeck, M. (2009) Growth related zonations in authigenic and hydrothermal quartz characterized by SIMS-, EPMA-, SEM-CL- and SEM-CC-imaging. Mineralogical Magazine, 73 (4) 633-643 doi:10.1180/minmag.2009.073.4.633
Korsakov, Andrey V., Perraki, Maria, Zhukov, Vladimir P., De Gussem, Kris, Vandenabeele, Peter, Tomilenko, Anatoly A. (2010) Is quartz a potential indicator of ultrahigh-pressure metamorphism? Laser Raman spectroscopy of quartz inclusions in ultrahigh-pressure garnets. European Journal of Mineralogy, 21 (6) 1313-1323 doi:10.1127/0935-1221/2009/0021-2006
Thompson, R. M., Downs, R. T. (2010) Packing systematics of the silica polymorphs: The role played by O-O nonbonded interactions in the compression of quartz. American Mineralogist, 95 (1) 104-111 doi:10.2138/am.2010.3241
Seifert, W., Rhede, D., Thomas, R., Förster, H.-J., Lucassen, F., Dulski, P., Wirth, R. (2011) Distinctive properties of rock-forming blue quartz: inferences from a multi-analytical study of submicron mineral inclusions. Mineralogical Magazine, 75 (4) 2519-2534 doi:10.1180/minmag.2011.075.4.2519
Henn, Ulrich, Schultz-Güttler, Rainer (2012) Review of some current coloured quartz varieties. The Journal of Gemmology, 33 (1) 29-43 doi:10.15506/jog.2012.33.1.29
Zhang, Siting, Liu, Yun (2014) Molecular-level mechanisms of quartz dissolution under neutral and alkaline conditions in the presence of electrolytes. Geochemical Journal, 48 (2) 189-205 doi:10.2343/geochemj.2.0298
Eder, S. D., Fladischer, K., Yeandel, S. R., Lelarge, A., Parker, S. C., Søndergård, E., Holst, B. (2015) A Giant Reconstruction of α-quartz (0001) Interpreted as Three Domains of Nano Dauphine Twins. Scientific Reports, 5. doi:10.1038/srep14545
Frelinger, Stefanie N., Ledvina, Matthew D., Kyle, J. Richard, Zhao, Donggao (2015) Scanning electron microscopy cathodoluminescence of quartz: Principles, techniques and applications in ore geology. Ore Geology Reviews, 65. 840-852 doi:10.1016/j.oregeorev.2014.10.008
Momma, Koichi, Nagase, Toshiro, Kuribayashi, Takahiro, Kudoh, Yasuhiro (2015) Growth history and textures of quartz twinned in accordance with the Japan law. European Journal of Mineralogy, 27 (1) 71-80 doi:10.1127/ejm/2014/0026-2411
Lin, Xiayang, Heaney, Peter J. (2017) Causes of Iridescence in Natural Quartz. Gems & Gemology, 53 (1). 68-81 doi:10.5741/gems.53.1.68
Glazer, A. M. (2018) Confusion over the description of the quartz structure yet again. Journal of Applied Crystallography, 51 (3). 915-918 doi:10.1107/s160057671800434x
Stalder, Roland (2021) OH point defects in quartz – a review. European Journal of Mineralogy, 33 (2) 145-163 doi:10.5194/ejm-33-145-2021
Farfan, Gabriela A., Rakovan, John, Ackerson, Michael R., Andrews, Benjamin J., Post, Jeffrey E. (2021) The origin of trapiche-like inclusion patterns in quartz from Inner Mongolia, China. American Mineralogist, 106 (11) 1797-1808 doi:10.2138/am-2021-7454
Murri, Mara, Prencipe, Mauro (2021) Anharmonic Effects on the Thermodynamic Properties of Quartz from First Principles Calculations. Entropy, 23 (10) 1366pp. doi:10.3390/e23101366
Sun, Liang, Zhang, Huan, Guan, Zanyang, Yang, Weiming, Zhang, Youjun, Sekine, Toshimori, Duan, Xiaoxi, Wang, Zhebin, Yang, Jiamin (2021) Sound Velocity Measurement of Shock-Compressed Quartz at Extreme Conditions. Minerals, 11 (12) 1334 doi:10.3390/min11121334
Shah, Sajjad Ahmad, Shao, Yongjun, Zhang, Yu, Zhao, Hongtao, Zhao, Lianjie (2022) Texture and Trace Element Geochemistry of Quartz: A Review. Minerals, 12 (8) 1042 doi:10.3390/min12081042
Significant localities for Quartz
Showing 126 significant localities out of 74,450 recorded on mindat.org.
Warning: mysqli::query(): (HY000/3024): Query execution was interrupted, maximum statement execution time exceeded in /home/mindat/www/show_class.php on line 5120
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
Struck out - Mineral was erroneously reported from this locality.
Faded * - Never found at this locality but inferred to have existed at some point in the past (e.g. from pseudomorphs).
All localities listed without proper references should be considered as questionable.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Afghanistan | |
| Ikram Mineralogy |
Argentina | |
| [var: Citrine] Raúl Jorge Tauber Larry´s collection. |
Australia | |
| [var: Citrine] Patrick Gundersen |
| [var: Amethyst] McColl (2002) |
| Bottrill (2018) |
| M Latham Collection |
| [var: Smoky Quartz] Bottrill et al. (2008) |
| R Bottrill +1 other reference | |
Austria | |
| [var: Amethyst] Kandutsch (2000) |
| [var: Smoky Quartz] Wachtler |
| [var: Smoky Quartz] Niedermayr et al. (1995) | |
| [var: Rutilated Quartz] Rudolf Hasler Collection | |
| Niedermayr et al. (1995) |
| [var: Rock Crystal] G. Niedermayr: Carinthia II 184./104.:254-255 (1994) | |
| Gerd Stefanik |
| Rudolf Hasler Collection |
| [var: Amethyst] Dr. H. Weninger (1976) |
| [var: Rock Crystal] Niedermayr et al. (1995) |
| Rudolf Hasler +1 other reference | |
| Alker (1975) +1 other reference |
| [var: Amethyst] Christian Bracke Collection / Mineralien Welt 15 (6) +1 other reference |
| [var: Amethyst] Lapis 29 (9) |
Belgium | |
| [var: Rock Crystal] Harjo Neutkens collection |
| [var: Rock Crystal] Hatert et al. (2002) |
| Hatert et al. (2002) |
Bolivia | |
| Collections of Alfredo Petrov and Dr. ... |
| [var: Amethyst] Alfredo Petrov |
| [var: Amethyst] Betts (n.d.) |
| [var: Amethyst] Josep Sanchez-Lafuente collection. | |
Bosnia and Herzegovina | |
| Faris Musija +1 other reference |
Brazil | |
| [var: Rose Quartz] - (n.d.) |
| RSA MINERAIS | |
| [var: Amethyst] Sauer (1982) +2 other references |
| [var: Amethyst] Sauer (1982) +1 other reference | |
Bulgaria | |
| [var: Amethyst] Ivan Pojarevski (bulgarianminerals.com) |
Canada | |
| Ann P. Sabina Rocks and Minerals for ... |
| Robinson et al. (1992) |
| Gait et al. (1990) +1 other reference |
| [var: Amethyst] Matthew Neuzil Collection |
| [var: Amethyst] Ontario Geological Survey Miscellaneous ... +5 other references | |
| [var: Amethyst] Rocks & Minerals (xxxx) +1 other reference |
| Ontario Gem Company | |
China | |
| [var: Amethyst] Moore (2006) |
| [Chalcedony var: Agate] Woodside (n.d.) |
Colombia | |
| Saenz (2005) |
Ecuador | |
| [var: Prase] Alejandro Felix Gutierrez |
France | |
| G. Signorelli |
| Mallet-Bachellier +7 other references |
| [var: Smoky Quartz] F. Gonnard (1906) |
| F. GONNARD (1906) | |
| [var: Amethyst] 207433 +2 other references |
| Favreau G. et al. (1996) |
| Harjo |
| Alain Steinmetz and Thierry Brunsperger ... |
| Aufschluss 1/85 |
| Rostan (2002) |
| Thierry JEAN |
Hungary | |
| Szakáll et al. (1996) |
India | |
| [var: Amethyst] Thomas P. Moore The Mineralogical ... |
Indonesia | |
| [var: Grape agate] Ivey (2018) |
Ireland | |
| O’Reilly et al. (1997) |
| [var: Amethyst] Nicholson (1847) |
| Barry Flannery Collection |
| Stephen Moreton |
| Barry Flannery (Personal Communication) |
| Barry Flannery (Personal Observation) +1 other reference |
| [var: Smoky Quartz] R Lawson & S Moreton Communication |
Italy | |
| Barelli (1835) +2 other references |
| - (n.d.) |
| Gambari (1868) +9 other references |
| Bombicci (1874) +1 other reference | |
| Bombicci (1869) | |
| Bombicci (1874) +2 other references |
| De Michele (1974) |
| [var: Amethyst] Olimpo (1981) +3 other references |
| [var: Amethyst] Torti (1973) +5 other references |
| [var: Smoky Quartz] |
| [var: Amethyst] Berg (1982) |
| Alessandro Genazzani collection |
| Giuliano bettini collection +2 other references | |
| |
| Orlandi P. et al. (LU) |
| [var: Smoky Quartz] Baldi M. |
| Gnoli +18 other references |
Kazakhstan | |
| [var: Amethyst] M. Chinellato +2 other references |
| [Chalcedony var: Agate] Bespaev et al. (2001) |
Kenya | |
| [var: Amethyst] Moore (2010) |
Madagascar | |
| [var: Amethyst] Moore (2001) +1 other reference |
| [var: Amethyst] www.madaquartz.com (2008) |
Mexico | |
| [var: Amethyst] Betts (n.d.) +1 other reference |
| [var: Amethyst] Kipfer (1974) +1 other reference | |
| [var: Amethyst] Wilson et al. (2004) |
| [var: Amethyst] Betts (n.d.) |
Morocco | |
| [var: Amethyst] Jordi Fabre [Pers. Com. 2009] +2 other references |
Namibia | |
| [var: Amethyst] [www.thamesvalleyminerals.com] +1 other reference |
| [var: Amethyst] Peter Seroka collection |
Nepal | |
| Calonge (2011) |
Niger | |
| [var: Amethyst] Sylvain Leroux information |
Norway | |
| Revheim (2006) +1 other reference |
| [var: Amethyst] Moløkken (1997) +2 other references |
| Álvaro Chicote Collection |
| [var: Amethyst] Moløkken (1990) |
| [var: Amethyst] Nordrum et al. (1997) |
Peru | |
| Econ Geol (1985) +1 other reference |
| collections of Rock Currier +3 other references |
| Mi.Rec. 28 (1997) |
Poland | |
| Schumacher K. (1878) |
Portugal | |
| Leal Gomes et al. (2009) |
Romania | |
| [var: Amethyst] Lapis 26 (9) +2 other references |
| [var: Amethyst] Clain et al. (2006) +1 other reference |
Russia | |
| [var: Amethyst] Oleg Lopatkin +1 other reference |
| [var: Amethyst] Lieber (1994) +1 other reference |
| [Chalcedony var: Agate] Godovikov et al. (1987) +4 other references |
| [var: Ferruginous Quartz] Amir Akhavan |
| [World of Stones 2:93] +2 other references |
| [var: Rock Crystal] [World of Stones 2/93 p.35] |
Slovakia | |
| [var: Amethyst] Ozdín et al. (2016) +1 other reference |
| [Chalcedony var: Agate] Ozdín D. & Števko M. |
| Slavomir ŠIMKO |
Slovenia | |
| Matija Vukovski Collection |
South Africa | |
| [var: Milky Quartz] Paul Meulenbeld collection Photo ID: ... |
| [var: Amethyst] www.mindat.org/mesg-55-48596.html +1 other reference |
South Korea | |
| [var: Amethyst] Lieber (1994) +1 other reference |
Spain | |
| Calvo (2016) +1 other reference |
| [var: Citrine] Arroyo et al. (1995) |
| [var: Amethyst] Curtó +3 other references |
| Calvo (2016) |
| [var: Smoky Quartz] Calvo et al. (2009) |
| [var: Amethyst] Calvo (1996) +1 other reference |
| Calvo (2016) |
| Casanova Honrubia +1 other reference |
Switzerland | |
| [var: Smoky Quartz] Stalder (1964) |
| [var: Rock Crystal] Altmann (1751) +6 other references |
| [var: Smoky Quartz] Stalder et al. (1998) | |
| Jahn (2004) |
| Taddei (1937) +3 other references |
Turkey | |
| [Chalcedony] Agricola (1546) |
Uruguay | |
| [var: Amethyst] Betts (n.d.) +1 other reference |
USA | |
| Min Rec 35:5 pp383-404 |
| Smith (1997) |
| Jake Harper: Field work |
| [var: Amethyst] Calzia +1 other reference |
| Ron Layton Collection |
| [var: Amethyst] Rocks & Min.: 59:11. |
| [Chalcedony] Personally collected by Donald Gilbert ... | |
| [var: Milky Quartz] Eckel et al. (1997) |
| Rowan M. Lytle +1 other reference |
| [var: Amethyst] Kenneth Holt specimen (locality info corrected courtesy of John Betts) +2 other references |
| Wolfe et al. (1960) |
| Moritz (n.d.) |
| Januzzi et al. (1976) | |
| Moritz (n.d.) |
| Januzzi et al. (1976) |
| Bill Barrett Coll. +1 other reference |
| Rowan M. Lytle Collection |
| [var: Smoky Quartz] Davis (1901) +2 other references |
| Moritz (n.d.) +1 other reference | |
| Williams (circa 1945 and 1899) +1 other reference | |
| USGS Professional Paper 255: 333-338. +5 other references |
| Specimens collected by Jeremy Zolan in ... +1 other reference |
| Moritz (n.d.) | |
| Powell (1987) |
| [var: Smoky Quartz] Vener (1987) | |
| [var: Amethyst] Brace (1823) | |
| Bill Barrett collection | |
| Weber et al. (1995) |
| A. Berluti collection +1 other reference |
| Zodac (1948) |
| Ague (1995) +1 other reference |
| [var: Amethyst] Moritz (n.d.) | |
| [var: Amethyst] Moritz (n.d.) |
| [var: Amethyst] Clark (2001) | |
| [var: Smoky Quartz] Wells (1887) |
| [var: Amethyst] Moore (2005) |
| Ted Johnson collection |
| T. Kennedy collection |
| [var: Rose Quartz] Barry Heath and Frank Perham +1 other reference |
| [var: Rose Quartz] Cameron +2 other references | |
| Rocks & Min.: 62: 443 +2 other references |
| [var: Rose Quartz] Stan Perham personal communication |
| [var: Rose Quartz] King et al. (6) |
| [var: Rose Quartz] Mineralogical Record 22:382 +1 other reference | |
| Mineralogical Record 22:382 +6 other references |
| [var: Rose Quartz] King et al. (1994) |
| [var: Amethyst] Mineralogical Record (1990) +1 other reference |
| [var: Amethyst] Mineralogical Record (1990) |
| Edith Trebilcock |
| |
| William Prescott (1852) |
| [var: Amethyst] Harvard Museum of Natural History +1 other reference |
| Gleba (2008) | |
| [var: Amethyst] Michael W. Kieron collection +1 other reference |
| [var: Amethyst] Betts (n.d.) |
| [Chalcedony var: Agate] Rosemeyer |
| Heinrich et al. (2004) | |
| Heinrich et al. (2004) |
| [Chalcedony var: Agate] Carl Dietrich |
| [Chalcedony var: Moss Agate] The River Runs North - the Story of Montana Moss Agate by Tom Harmon (author) |
| Ryan Sweeney Collection |
| [var: Amethyst] Rocks & Minerals 47:3 pp160-164 +1 other reference |
| [var: Amethyst] Rob Lavinsky +1 other reference |
| [var: Sceptre Quartz] Crystal Tips #1 +2 other references | |
| Randy Lahey |
| [var: Pseudocubic Quartz] Tarr et al. (1929) |
| Min Rec 22:5 pp359-366 The Smoky Bear ... |
| Dana 7:I:592. +1 other reference |
| Econ Geol (1990) |
| [var: Amethyst] www.grandfather.com/museum/amethyst.htm ... +1 other reference |
| pg. 234 +1 other reference |
| [var: Amethyst] Miller (1971) |
| [var: Amethyst] Mineralogical Record (1990) |
| Rocks & Minerals (1986) +1 other reference |
| Rocks & Min.: 17:51 +4 other references |
| [var: Amethyst] [Rakovan et al +3 other references |
| Miller (1971) |
| [var: Amethyst] Betts (n.d.) |
| [var: Rose Quartz] Rocks & Min.: 10:145 +2 other references |
| [var: Rose Quartz] Smith et al. (2000) | |
| Calkins +3 other references |
| Matthew Lambert |
| Linda D. Gill +3 other references |
| Min Record:20 (5) +2 other references |
| [var: Amethyst] UBC specimen |
| Eric He's Collection +1 other reference |
| Cannon (1975) |
| [var: Amethyst] Bob Jackson +1 other reference | |
| Rocks and Minerals 66:6 +1 other reference | |
Vietnam | |
| "Mario Lazzerini Denchi' Collection" |
Zambia | |
| [var: Citrine] Peter Beckwith collection |
Quick NavTopMacro- and Cryptocrystalline QuartzQuartz VarietiesStructure of QuartzHandedness of Quartz CrystalsMorphologyColored Quartz VarietiesOccurrence of QuartzIdentificationID Requirements on MindatHandling QuartzAbout QuartzUnique IdentifiersIMA Classification Classification Mineral SymbolsPronunciation Physical Properties Optical Data Chemistry Age distributionCrystallography Crystallographic forms Crystal StructureX-Ray Powder DiffractionGeological EnvironmentSynonymsOther LanguagesVarietiesCommon AssociatesStrunz-MindatOther InformationQuartz in petrologyInternet Links References Significant localities


















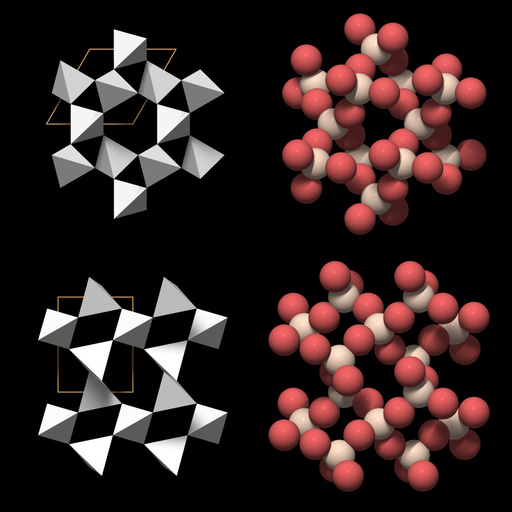
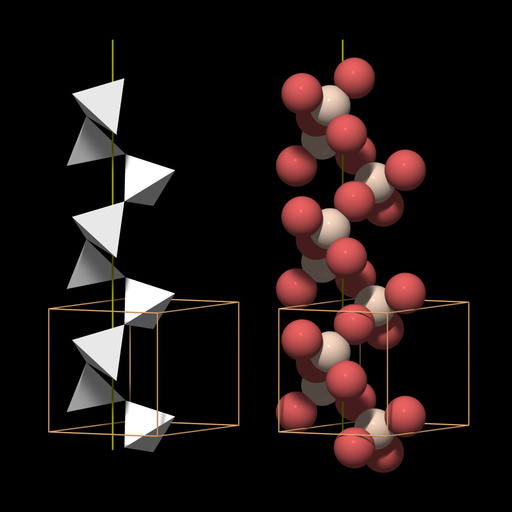
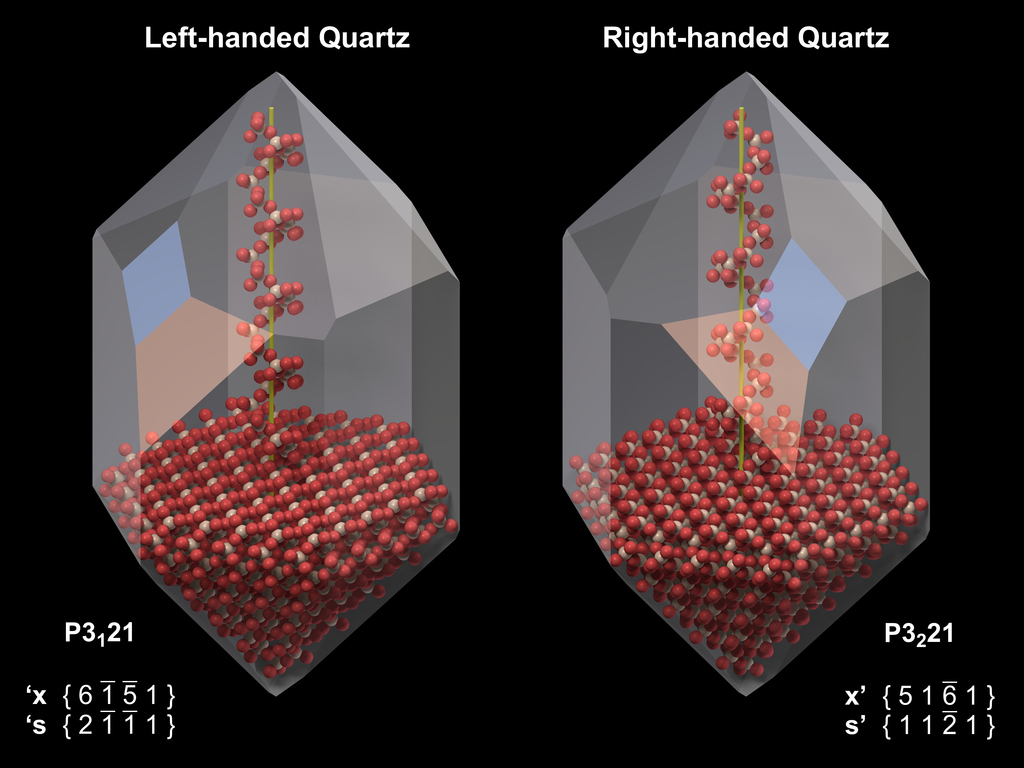
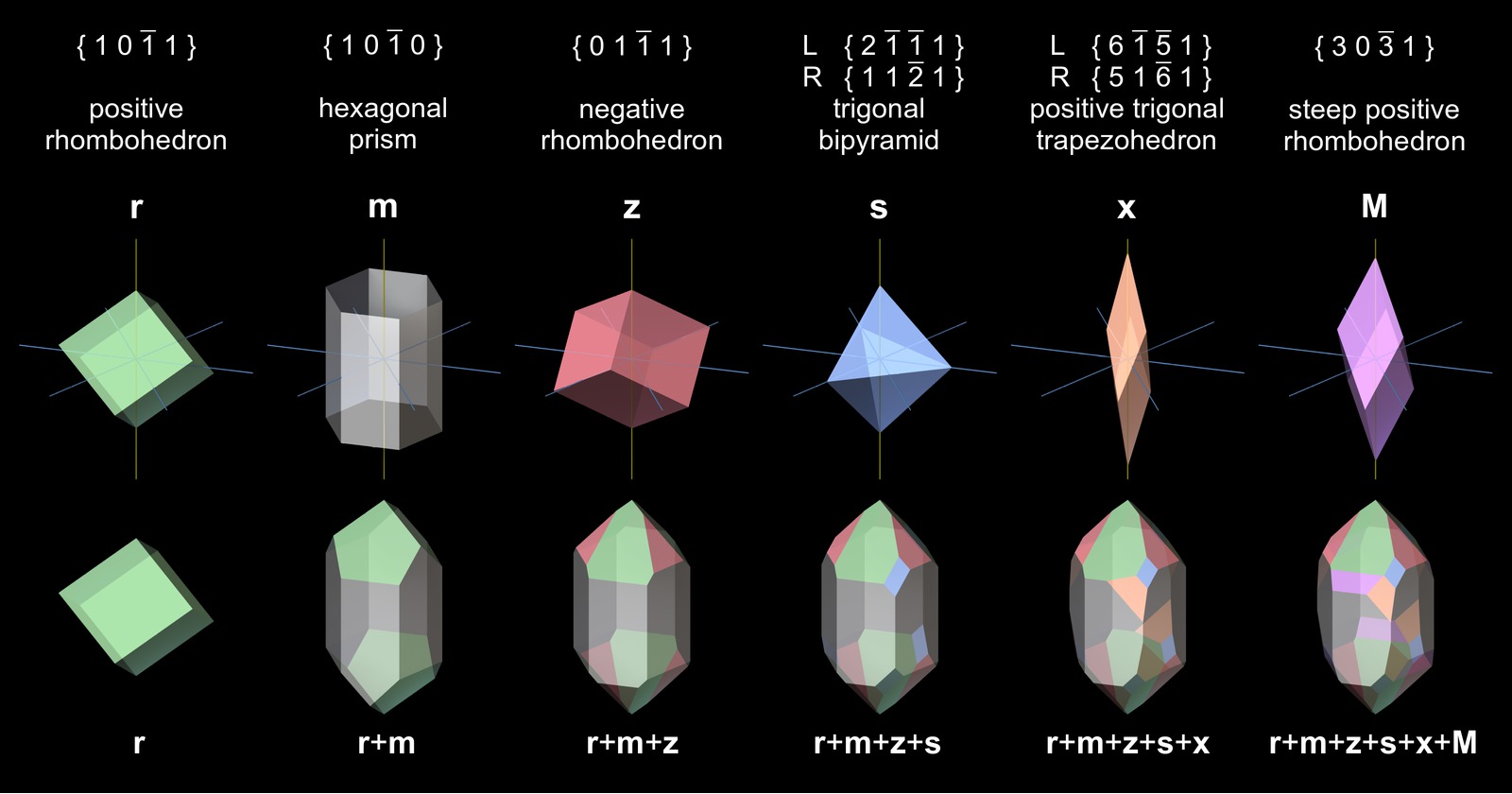
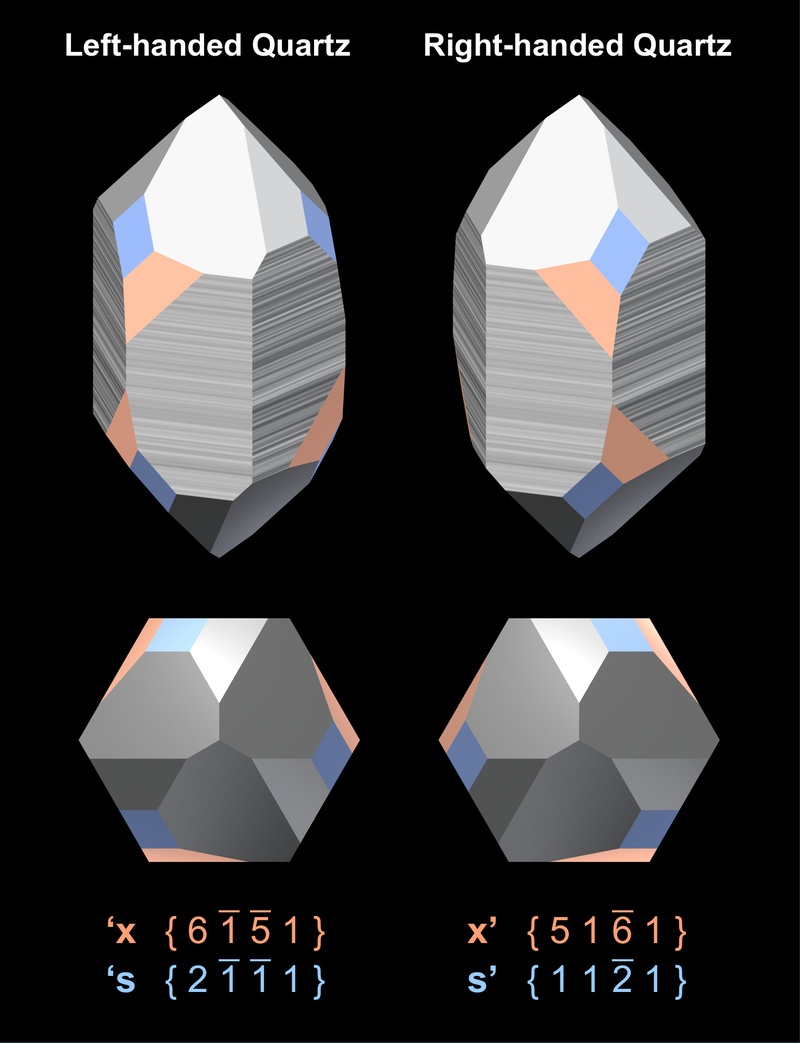
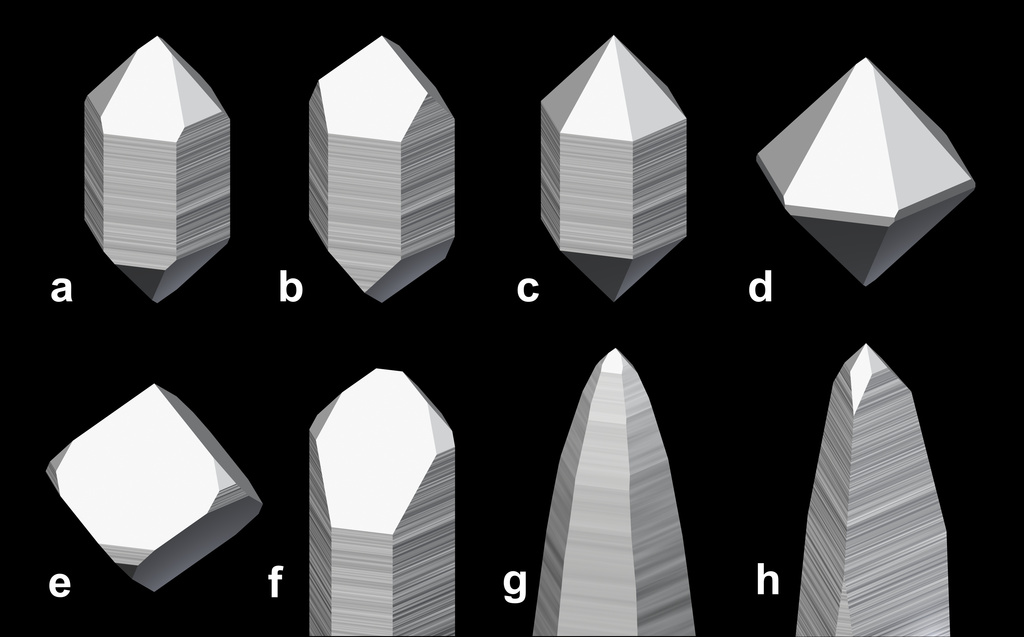



























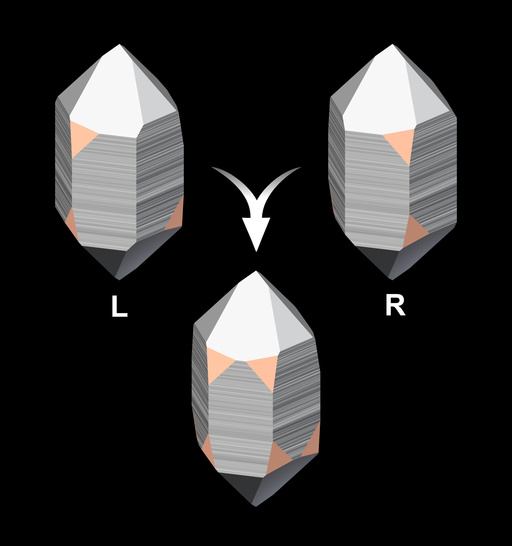









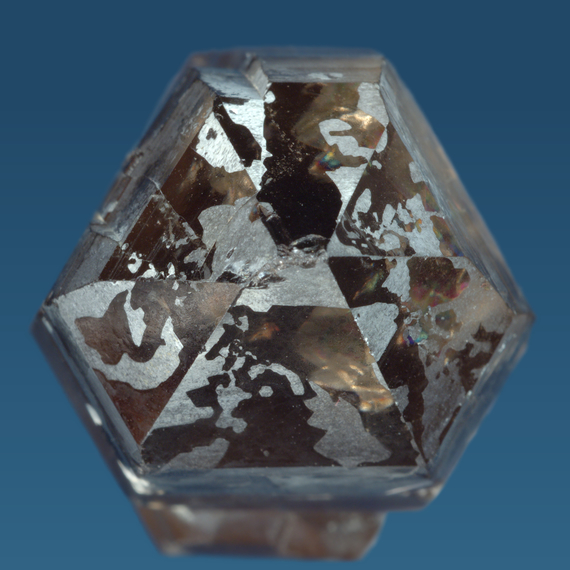






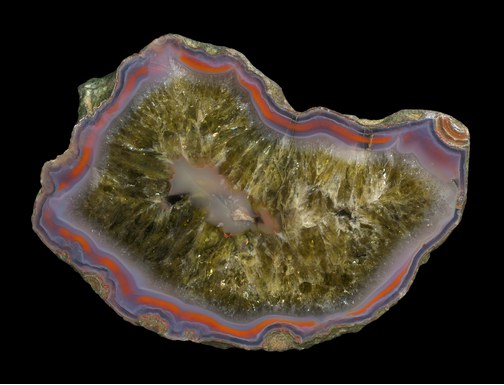



























Las Vigas de Ramírez Municipality, Veracruz, Mexico