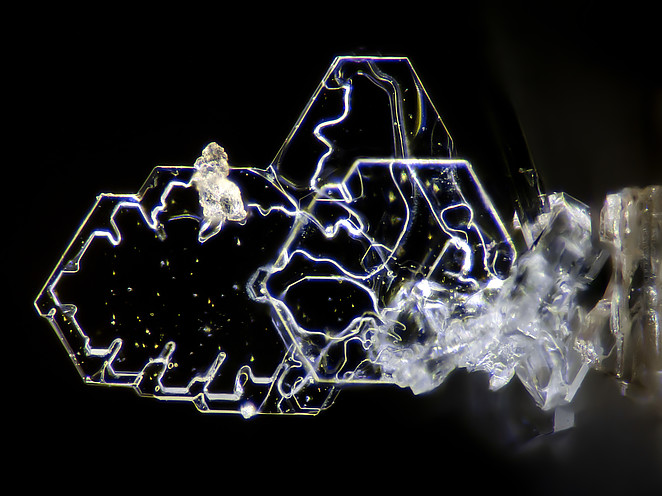
Tridymite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Tridymite
Formula:
SiO2
Colour:
Colourless, white, yellowish white, or grey
Lustre:
Vitreous
Hardness:
6½ - 7
Specific Gravity:
2.25 - 2.28
Crystal System:
Triclinic
Name:
From the Greek "Tridymos", triplet, alluding to its common twinning as trillings.
Type Locality:
Polymorph of:
Tridymite is a low pressure, mostly high-temperature-stable polymorph of silica that can also form or persist metastably at low temperatures. The high-temperature form occurs most notably as vapour-deposited, platey crystals in vesicles in some volcanic rocks, also rarely as phenocrysts in some felsic volcanics, or as a contact metamorphic material in some hornfels.
Tridymite can occur in seven polytypes and the most common at standard atmospheric pressure are known as α and β; both commensurately and incommensurately modulated structure variants are known. Below 100°C the triclinic form, α-tridymite is stable; there are also orthorhombic, monoclinic and hexagonal polytypes stable at higher temperatures. The orthorhombic β-tridymite polytype is most stable at elevated temperatures (>870°C) and it converts to β-cristobalite above 1470°C. However, tridymite does not usually form directly from pure β-quartz, and it is usually stabilised by alkali metals. Otherwise β-quartz transitions directly to cristobalite at 1050°C without the occurrence of the tridymite phase.
Some polytypes of tridymite ( from Deer et al. 2004)
In the above table, M, O, H, C, P, L and S stand for monoclinic, orthorhombic, hexagonal, centered, primitive, low (temperature) and superlattice. T indicates the temperature, at which the corresponding phase is relatively stable, though the interconversions between phases are complex and sample dependent, and all these forms can coexist at ambient conditions. Mineralogy handbooks often arbitrarily assign tridymite to the triclinic crystal system, yet use hexagonal Miller indices because of the hexagonal crystal shape.
A low temperature form is commonly reported as a constituent of certain types of opal, intergrown with cristobalite (opal-CT), found in many environments including marine sedimentary rocks derived from biogenic opaline sediments, and low temperature cavity infillings, replacements, etc., including some precious opal (Sanders, 1975). Some wood opal is mostly tridymite (Mitchell & Tufts, 1973). However, some workers note that opal is hydrous and lacks any long-range ordering, the opal structure just mimicking cristobalite and tridymite, so may not contain true tridymite (Smith, 1998).
Large tridymite deposits have been detected on Mars, but their nature and origin are uncertain (Lakdawalla, 2015).
Tridymite can occur in seven polytypes and the most common at standard atmospheric pressure are known as α and β; both commensurately and incommensurately modulated structure variants are known. Below 100°C the triclinic form, α-tridymite is stable; there are also orthorhombic, monoclinic and hexagonal polytypes stable at higher temperatures. The orthorhombic β-tridymite polytype is most stable at elevated temperatures (>870°C) and it converts to β-cristobalite above 1470°C. However, tridymite does not usually form directly from pure β-quartz, and it is usually stabilised by alkali metals. Otherwise β-quartz transitions directly to cristobalite at 1050°C without the occurrence of the tridymite phase.
Some polytypes of tridymite ( from Deer et al. 2004)
|
In the above table, M, O, H, C, P, L and S stand for monoclinic, orthorhombic, hexagonal, centered, primitive, low (temperature) and superlattice. T indicates the temperature, at which the corresponding phase is relatively stable, though the interconversions between phases are complex and sample dependent, and all these forms can coexist at ambient conditions. Mineralogy handbooks often arbitrarily assign tridymite to the triclinic crystal system, yet use hexagonal Miller indices because of the hexagonal crystal shape.
A low temperature form is commonly reported as a constituent of certain types of opal, intergrown with cristobalite (opal-CT), found in many environments including marine sedimentary rocks derived from biogenic opaline sediments, and low temperature cavity infillings, replacements, etc., including some precious opal (Sanders, 1975). Some wood opal is mostly tridymite (Mitchell & Tufts, 1973). However, some workers note that opal is hydrous and lacks any long-range ordering, the opal structure just mimicking cristobalite and tridymite, so may not contain true tridymite (Smith, 1998).
Large tridymite deposits have been detected on Mars, but their nature and origin are uncertain (Lakdawalla, 2015).
Unique Identifiers
Mindat ID:
4015
Long-form identifier:
mindat:1:1:4015:6
GUID
(UUID V4):
(UUID V4):
a6690284-91bd-4ecf-a045-8f91efc49d49
IMA Classification of Tridymite
Approved, 'Grandfathered' (first described prior to 1959)
First published:
1868
Classification of Tridymite
4.DA.10
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
D : Metal: Oxygen = 1:2 and similar
A : With small cations: Silica family
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
D : Metal: Oxygen = 1:2 and similar
A : With small cations: Silica family
75.1.2.1
75 : TECTOSILICATES Si Tetrahedral Frameworks
1 : Si Tetrahedral Frameworks - SiO2 with [4] coordinated Si
75 : TECTOSILICATES Si Tetrahedral Frameworks
1 : Si Tetrahedral Frameworks - SiO2 with [4] coordinated Si
7.8.3
7 : Oxides and Hydroxides
8 : Oxides of Si
7 : Oxides and Hydroxides
8 : Oxides of Si
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Trd | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Trd | Kretz (1983) | Kretz, R. (1983) Symbols of rock-forming minerals. American Mineralogist, 68, 277–279. |
| Trd | Siivolam & Schmid (2007) | Siivolam, J. and Schmid, R. (2007) Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: List of mineral abbreviations. Web-version 01.02.07. IUGS Commission on the Systematics in Petrology. download |
| Trd | Whitney & Evans (2010) | Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 doi:10.2138/am.2010.3371 |
| Trd | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
| Trd | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Physical Properties of Tridymite
Vitreous
Transparency:
Transparent, Translucent
Comment:
May be pearly on {0001}
Colour:
Colourless, white, yellowish white, or grey
Streak:
White
Hardness:
6½ - 7 on Mohs scale
Tenacity:
Brittle
Cleavage:
Poor/Indistinct
[0001] Indistinct, [1010] Imperfect
[0001] Indistinct, [1010] Imperfect
Fracture:
Conchoidal
Density:
2.25 - 2.28 g/cm3 (Measured) 2.28 g/cm3 (Calculated)
Optical Data of Tridymite
Type:
Biaxial (+)
RI values:
nα = 1.468 - 1.482 nβ = 1.470 - 1.484 nγ = 1.474 - 1.486
2V:
Measured: 40° to 86°, Calculated: 50° to 72°
Max Birefringence:
δ = 0.006

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Moderate
Dispersion:
none
Chemistry of Tridymite
Mindat Formula:
SiO2
Elements listed:
Crystallography of Tridymite
Crystal System:
Triclinic
Class (H-M):
1 - Pedial
Cell Parameters:
a = 9.932(5) Å, b = 17.216(6) Å, c = 81.854(9) Å
α = 90°, β = 90°, γ = 90°
α = 90°, β = 90°, γ = 90°
Ratio:
a:b:c = 0.577 : 1 : 4.755
Unit Cell V:
13,996.16 ų (Calculated from Unit Cell)
Z:
320
Morphology:
Pseudohexagonal plates, wedge-shaped, tabular.
Twinning:
Trilling, multiple contact twins or simple twins on {1016} and contact or penetration twins on {3034}
Comment:
Orthorhombic, pseudohexagonal; triclinic below 100°C
Crystallographic forms of Tridymite
Crystal Atlas:
Image Loading
3d models and HTML5 code kindly provided by
www.smorf.nl.
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0000531 | Tridymite | Dollase W A, Baur W H (1976) The superstructure of meteoritic low tridymite solved by computer simulation American Mineralogist 61 971-978 |  | 1976 | Steinbach meteorite, Saxony, Germany | 0 | 293 |
| 0006615 | Tridymite | Graetsch H, Topalovic-Dierdorf I (1996) 29Si MAS NMR spectrum and superstructure of modulated tridymite L3-To(MX-1) European Journal of Mineralogy 8 103-113 | 1996 | synthetic | 0 | 293 | |
| 0006616 | Tridymite | Graetsch H, Topalovic-Dierdorf I (1996) 29Si MAS NMR spectrum and superstructure of modulated tridymite L3-To(MX-1) European Journal of Mineralogy 8 103-113 | 1996 | synthetic | 0 | 293 | |
| 0008552 | Tridymite | Graetsch H (2001) X-ray powder diffraction study on the modulated high temperature forms of SiO2 tridymite between 110 and 220 C Physics and Chemistry of Minerals 28 313-321 | 2001 | synthetic | 0 | 293 | |
| 0008553 | Tridymite | Graetsch H (2001) X-ray powder diffraction study on the modulated high temperature forms of SiO2 tridymite between 110 and 220 C Physics and Chemistry of Minerals 28 313-321 | 2001 | synthetic | 0 | 388 | |
| 0020733 | Tridymite | Dollase W A (1967) The crystal structure at 220 C of orthothombic high tridymite from the Steinbach meteorite Acta Crystallographica 23 617-623 |  | 1967 | Steinbach meteorite | 0 | 293 |
| 0020734 | Tridymite | Kato V K, Nukui A (1976) Die kristallstruktur des monoklinen tief-tridymits Acta Crystallographica B32 2486-2491 |  | 1976 | synthetic | 0 | 293 |
| 0009625 | Tridymite | Konnert J H, Appleman D E (1978) The crystal structure of low tridymite Acta Crystallographica B34 391-403 |  | 1978 | Plumas County, California, USA | 0 | 293 |
| 0020744 | Tridymite | Lee S, Xu H (2019) Using powder XRD and pair distribution function to determine anisotropic atomic displacement parameters of orthorhombic tridymite and tetragonal cristobalite Acta Crystallographica B75 https://doi.org/10.1107/S2052520619000933 | 2019 | rhyolitic rock, New Mexico, USA | 0 | 293 | |
| 0013132 | Tridymite | Hirose T, Kihara K, Okuno M, Fujinami S, Shinoda K (2005) X-ray, DTA and Raman studies of monoclinic tridymite and its higher temperature orthorhombic modification with varying temperature. Journal of Mineralogical and Petrological Sciences 100 55-69 |  | 2005 | synthetic | 0 | 298 |
| 0013133 | Tridymite | Hirose T, Kihara K, Okuno M, Fujinami S, Shinoda K (2005) X-ray, DTA and Raman studies of monoclinic tridymite and its higher temperature orthorhombic modification with varying temperature. Journal of Mineralogical and Petrological Sciences 100 55-69 |  | 2005 | synthetic | 0 | 373 |
| 0013134 | Tridymite | Hirose T, Kihara K, Okuno M, Fujinami S, Shinoda K (2005) X-ray, DTA and Raman studies of monoclinic tridymite and its higher temperature orthorhombic modification with varying temperature. Journal of Mineralogical and Petrological Sciences 100 55-69 |  | 2005 | synthetic | 0 | 413 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 4.30 Å | (100) |
| 4.09 Å | (90) |
| 3.80 Å | (60) |
| 3.249 Å | (30) |
| 2.964 Å | (16) |
| 2.483 Å | (16) |
| 2.305 Å | (8) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 1: Primary nebular phases | 4.567-4.561 |
| 4 : Primary chondrule phases | 4.566–4.561 |
| Stage 2: Planetesimal differentiation and alteration | 4.566-4.550 |
| 5 : Primary asteroid phases | 4.566–4.560 |
| Stage 3a: Earth’s earliest Hadean crust | >4.50 |
| 9 : Lava/xenolith minerals (hornfels, sanidinite facies) | |
| 10 : Basalt-hosted zeolite minerals | |
| Stage 4a: Earth’s earliest continental crust | >4.4-3.0 |
| 20 : Acidic volcanic rocks | |
| Near-surface Processes | |
| 26 : Hadean detrital minerals | |
| Stage 10a: Neoproterozoic oxygenation/terrestrial biosphere | <0.6 |
| 50 : Coal and/or oil shale minerals | <0.36 |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 54 : Coal and other mine fire minerals (see also #51 and #56) |
Geological Setting:
Vapor phase deposition in vesicles and lithophysae, phenocrysts in volcanic rocks, contact metamorphosed sandstones.
Type Occurrence of Tridymite
Reference:
vom Rath, G. (1868) Vorläufige Mitteilung über eine neue Kristallform der Kieselsäure (Tridymit). Annalen der Physik und Chemie: 209: 507.
Synonyms of Tridymite
Other Language Names for Tridymite
Varieties of Tridymite
| Christensenite | Name given to a solid solution of 5 % NaAlSiO4 in tridymite from a lava at Deception Island, Western Antartica found during the Norwegian Antartic expeditions 1927-1928. |
Common Associates
Associated Minerals Based on Photo Data:
| 257 photos of Tridymite associated with Hematite | Fe2O3 |
| 91 photos of Tridymite associated with Phlogopite | KMg3(AlSi3O10)(OH)2 |
| 78 photos of Tridymite associated with Fayalite | Fe2+2SiO4 |
| 73 photos of Tridymite associated with Pseudobrookite | Fe2TiO5 |
| 62 photos of Tridymite associated with Enstatite | Mg2Si2O6 |
| 49 photos of Tridymite associated with Titanite | CaTi(SiO4)O |
| 42 photos of Tridymite associated with Augite | (CaxMgyFez)(Mgy1Fez1)Si2O6 |
| 42 photos of Tridymite associated with Magnetite | Fe2+Fe3+2O4 |
| 42 photos of Tridymite associated with Edenite | NaCa2Mg5(Si7Al)O22(OH)2 |
| 34 photos of Tridymite associated with Quartz | SiO2 |
Related Minerals - Strunz-mindat Grouping
| 4.DA. | Chibaite | SiO2 · n(CH4, C2H6, C3H8, i-C4H10) (n = 3/17 (max)) |
| 4.DA. | Carbon Dioxide Ice | CO2 |
| 4.DA. | Bosoite | SiO2 · nCxH2x+2 |
| 4.DA.05 | Quartz | SiO2 |
| 4.DA.10 | Opal | SiO2 · nH2O |
| 4.DA.15 | Cristobalite | SiO2 |
| 4.DA.20 | Mogánite | SiO2 |
| 4.DA.25 | Melanophlogite | 46SiO2 · 6(N2,CO2) · 2(CH4,N2) |
| 4.DA.30 | Lechatelierite | SiO2 |
| 4.DA.35 | Coesite | SiO2 |
| 4.DA.40 | Stishovite | SiO2 |
| 4.DA.45 | Keatite | SiO2 |
| 4.DA.50 | Seifertite | SiO2 |
| 4.DA.55 | Quartz-beta | SiO2 |
Other Information
Thermal Behaviour:
High tridymite or β-tridymite forms between 870 and 1470°C.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Tridymite
mindat.org URL:
https://www.mindat.org/min-4015.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Tridymite
Reference List:
Cressey, G. (2004) W.A. Deer, R.A. Howie, W.S. Wise and J. Zussman. Rock-Forming Minerals. Volume 4B. Second Edition. Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites.
London (The Geological Society) 2004, xv + 982 pp. £125 (£62.50 to GSL members) ISBN 1-86239-144-0. Hardback. Mineralogical Magazine, 68 (5) 831-832 doi:10.1180/0680831p.22
Localities for Tridymite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Antarctica | |
| Ruzicka et al. (1999) |
| Meteorlogical Bulletin | |
| Izawa et al. (2010) |
| aaa.wustl.edu (2005) | |
| curator.jsc.nasa.gov (2013) |
| Ohgo et al. (2017) | |
| Browne |
| Collection of RJ Martin | |
| Barth et al. (1944) |
Argentina | |
| CRAVERO +1 other reference |
| ROBL +1 other reference |
Australia | |
| Rattigan (1967) |
| Lovering (1975) |
| Loughnan et al. (1960) |
| Garcia et al. (1978) |
| Birch (1999) |
| Ikeda et al. (1996) +1 other reference |
| Noble et al. (1983) |
| Floran et al. (1978) +2 other references |
| Bunch et al. (1970) +1 other reference |
| Bottrill et al. (2020) |
| Sorrell (n.d.) |
| Judy Rowe collection |
| Museum Victoria Mineralogy Collection | |
| Sorrell (n.d.) |
| Sorrell (n.d.) |
| Museum Victoria M 44321 |
| Birch et al. (2008) |
| Chennaoui Aoudjehane et al. (2007) |
| McCall et al. (1966) +2 other references |
| Mittlefehldt et al. (1998) |
Austria | |
| Schebesta (1983) +2 other references |
| Götzinger et al. (2009) |
| Niedermayr et al. (1995) |
| Dominik Sorger: „Mineralogical ... |
| Exel (1993) |
| W. Postl et al.: European Journal of Mineralogy 16 (2) |
| Postl et al. (1996) |
| Lapis 2003 (1) +1 other reference |
Azerbaijan | |
| rruff.geo.arizona.edu (n.d.) +1 other reference |
Bangladesh | |
| Powell (1971) +1 other reference |
Belarus | |
| Prinz et al. (1980) |
Belgium | |
| Blaß et al. (1995b) |
Bolivia | |
| Federico Ahlfeld y Jorge Muñoz Reyes (1955) |
| Kempff et al. (La Paz, 2009) | |
Brazil | |
| Steele et al. (1976) |
| Revista Brasileira de Geociências |
| Duke (1967) |
| Costa et al. (2016) |
Burundi | |
| Van Wambeke ( The Karonge rare earth deposits, Republic of Burundi: New mineralogical-geochemical data and origin of the mineralization. Mineralium Deposita. 12 ) |
Canada | |
| Kimura et al. (2005) |
| Friedlaender (1968) |
Chile | |
| with analyses of Vaca Muerta +2 other references |
China | |
| Rambaldi et al. (1986) |
| Jia Li et al. (1998) |
| Jia Li et al. (1998) | |
| Grossman (1997) |
| Tianhu Chen et al. (2002) |
| Querol +9 other references |
Costa Rica | |
| Rodríguez et al. (2017) |
Czech Republic | |
| Jirásek (2001) |
| Žáček V |
| Pauliš |
| Szakáll (2002) | |
Ecuador | |
| vom Rath (1872) |
El Salvador | |
| Torio-Henríquez (2007) |
Equatorial Guinea | |
| Thomé (1970) +6 other references |
Estonia | |
| Keil (1968) +1 other reference |
Faroe Islands | |
| Caucia et al. (2013) |
Finland | |
| Keil (1968) |
| Prinz et al. (1980) |
| Lehtinen et al. (toim.) |
France | |
| Bertwerth (1912) +1 other reference |
| Alfred Lacroix (1890's) |
| Varet (1970) +1 other reference |
| Fermis (2023) |
| Vernay R. (2005) |
| M. Christophe Michel-Lévy |
| Barrier D et al. (2004) |
| Müller et al. (2023) |
| In Collection micromounts Eric Naud ( FRANCE ) |
| Designolle (1996) | |
| Alain Tuel collection |
| P & E Médard collection | |
| Médard et al. (2021) | |
| Pierre Le Roch Collection |
| Valverde (2009) |
| Médard P. et al. (2009) |
| Lacroix (1910) |
| Prinz et al. (1980) |
Georgia | |
| Kekelia et al. (2017) |
Germany | |
| in the collection of Christof Schäfer |
| Grubenmann (1886) |
| Weiß (1990) |
| Wittern (2001) |
| Wittern (2001) |
| Weiß (1990) |
| Pöllmann et al. (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Betz (2019) |
| Müller (1967) |
| Collection of Walter Göttler |
| Koritnig (1955) |
| D. Pawlowski: "Mineralfundstellen im Sauerland" (Munich) |
| Schnorrer-Köhler (1986) +1 other reference |
| Blaß et al. (1995) |
| International Association of Collectors ... +1 other reference | |
| Rath et al. (1868) |
| vom Rath (1872) | |
| Rath et al. (1868) | |
| Brauns et al. (1922) | |
| Brauns et al. (1922) | |
| Ramdohr (1969) |
| Floran et al. (1978) +1 other reference |
| Hentschel (1975) |
| Weiß (1990) |
| Brauns (1922) |
| Streng (1871) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) | |
| Personally collected by Günter Frenz | |
| Lapis 1988 (7+8) | |
| Hentschel (1983) |
| Lehmann (1877) | |
| Hentschel (1983) |
| Brauns |
| collection C.&H. Schäfer |
| Leu (2017) |
| Hentschel (1983) |
| Hentschel (1983) | |
| Hentschel (1975) |
| Lehmann (1877) | |
| Lehmann (1877) |
| Christof Schäfer |
| Personally collected by Günter Frenz |
| Leu |
| Weiß (1990) |
| Personally collected by Günter Frenz |
| Schäfer |
| Guth et al. (Löley) | |
| Weiß (1990) |
| Blaß et al. (2012) |
| Weiß (1990) |
| Hentschel (1983) |
| Hentschel (1987) | |
| Wittern (2001) |
| Blaß (2020) |
| Schüller |
| Weiß (1990) |
| Wittern (2001) |
| Lapis (1) |
| Reid et al. (1973) +1 other reference |
| Tuček K.: Meteority a jejich výskyt v ... | |
| Witzke et al. (2012) |
| Witzke et al. (2012) |
| T. Witzke & T. Giesler (2001) |
| Witzke et al. (2012) | |
| Thomas et al. (2022) |
| WITZKE (2018) |
| T. Witzke & F. Rüger: Lapis 1998 (7/8) |
Greece | |
| Hanke (1996) |
| Hezel (2006) |
| Econ Geol (1989) |
Greenland | |
| Lindsley et al. (1969) |
Hungary | |
| Szakáll: Topographia Mineralogica ... |
| Szakáll: Topographia Mineralogica ... | |
| Szakáll & Jánosi : Minerals of Hungary | |
| Geoda Nr. 1 (2010) | |
| Szakáll-Weiszburg: Telkibánya |
| Szakáll et al. (1996) |
| Bulletin of the Hungarian Geological ... | |
| Collection of Gábor _Mesics |
| Szakáll et al. (1996) | |
| |
| Szakáll et al. (1996) |
| Szakáll et al. (1996) | |
| Szakáll et al. (1996) |
| Szakáll: Topographia Mineralogica ... |
| Szakáll: Topographia Mineralogica ... | |
| Szakáll: Mineralogica Topographia ... | |
| Szakáll et al. (1996) |
| geomania.hu (2011) |
| Society of Economic Geologists Student Chapter University of Miskolc (2018) | |
| Mineral Species of Hungary | |
| Szakáll | |
| Szakáll-Jánosi: Minerals of Hungary |
| |
| Szakáll et al. (2010) |
| www.mineral.hermuz.hu (2003) |
| Szakáll-Gatter-Szendrei:Mineral ... |
| Mineral Species of Hungary |
| Szakáll et al. (1996) |
| |
| Koch: Minerals of Hungary |
Iceland | |
| Found by Björn Mamat (inclusions in Obsidian) |
India | |
| Bridges et al. (1995) |
| |
Indonesia | |
| Sun et al. (2008) |
| G. Camus et al. (Indonesia) |
| Hartiningsih et al. (2022) |
Iran | |
| Powell (1971) +1 other reference |
Israel | |
| Britvin et al. (2019) |
Italy | |
| Bortolozzi (n.d.) |
| [vom Rath et al. (1872) +1 other reference |
| E. Passaglia et al. |
| Bortolozzi (n.d.) |
| Barrese et al. (1994) |
| Microprobe analysis by Victor Sharygin (Novosibirsk, Russia) |
| Burli et al. (2010) |
| forum.amiminerals.it (n.d.) | |
| Bortolozzi (n.d.) |
| Federico (1968) +1 other reference |
| in the collection of Luigi Mattei |
| Collected & analized by Rossano Carlini | |
| Bortolozzi (n.d.) | |
| De Michele (1974) +1 other reference |
| Bortolozi G. (1986) | |
| Sitta et al. (1983) |
| Urraci E.- Lini M. – Mattias P. (2006) |
| Gamboni et al. (2021) |
| Bortolozzi (n.d.) | |
| Boscardin. M. (1975) | |
| samples collected by Luigi Chiappino +1 other reference |
| Preite D. : I minerali etnei di Biancavilla (CT) +1 other reference |
| Bergeat (1900) |
| MERCALLI G. (1891) |
| Liotti L. (Grosseto) |
| Barsotti et al. (2006) |
| Wheeler et al. (1996) +1 other reference |
| Zorzi (2014) |
| Fabris et al. (2014) +1 other reference |
| Neubauer (2005) +1 other reference |
| Del Caldo et al. (1973) |
| Mattioli et al. (2008) +1 other reference |
| "Minerali del Vicentino - La natrolite" ... |
Japan | |
| Koga (1982) |
| Ibaraki et al. (1991) |
| S. Hamasaki (2002) |
| S. Hamasaki (2002) | |
| S. Hamasaki (2002) | |
| S. Hamasaki (2002) | |
| Miyashiro (1956) | |
| Petrov (n.d.) |
| |
| Sadanaga et al. (1974) +1 other reference |
| Sadanaga et al. (1974) |
| in the collection of Christof Schäfer |
| Petrov (n.d.) |
| Kato (2009) |
Jordan | |
| Galuskin et al. (2023) |
Kenya | |
| Schulze et al. (1994) |
Lebanon | |
| Kruszewski et al. (South Lebanon) |
Lithuania | |
| Bukovanská et al. (1991) +1 other reference |
Madagascar | |
| Lacroix (1922) | |
| Behier (1963) |
| Lacroix (1922) |
Malawi | |
| Mason (1966) |
Mauritania | |
| Mittlefehldt et al. (1998) |
Mexico | |
| |
| Panczner (1987) |
| [AmMin 85:263] |
| Am Min (1955) |
| Annalen der Physik und Chemie +1 other reference |
| Foshag et al. (1942) |
| Panczner (1987) |
| Sarafian et al. (2013) |
Middle East | |
| Vapnik et al. (2006) | |
Mongolia | |
| Bischoff (1993) +2 other references |
Morocco | |
| Luetcke (n.d.) |
Namibia | |
| von Bezing (2007) |
| Petaev et al. (1997, March) +1 other reference |
Netherlands | |
| T.G. Nijland |
| T.G. Nijland |
New Zealand | |
| Collection of RJ Martin |
| www.mightyriver.co.nz (n.d.) |
| Collection of RJ Martin |
| Martin Stolworthy Collection |
| Cole (1970) |
| Cody et al. (1979) | |
| Marshal (1893) +1 other reference |
| Hackett W R. |
| Lloyd et al. (1996) |
| Sutherland et al. (ed.) |
| Collection of RJ Martin correlated to ... |
| Brown et al. (1994) |
| Personal collection of Rod Martin |
| Judy Rowe collection |
| Collection of Rod Martin | |
| Collection of RJ Martin | |
| Thornton (Ed.) | |
| Collection of Rod Martin | |
| Collection of RJ Martin | |
| Essence of Microscope | |
| Collection of RJ Martin correlated to ... |
| Collection of RJ Martin correlated to ... | |
| Collection of RJ Martin correlated to ... | |
| Smith |
| Collection of RJ Martin correlated to ... |
| R. Martin. Tairua Essence of Microscope |
| Moore (1979) | |
| Collection of RJ Martin correlated to ... | |
Nicaragua | |
| Hynek et al. (2013) |
Nigeria | |
| Mason (1967) |
Northwest Africa Meteorites | |
| Meteoritical Bulletin et al. (2001) | |
| Srinivasan et al. (2018) | |
Oman | |
| The Meteoritical Bulletin |
Pakistan | |
| Keil (1968) |
| Brian Mason (1966) |
Panama | |
Papua New Guinea | |
| Mason et al. (1973) +1 other reference |
| Lowder (1970) |
Peru | |
| Dill et al. (1997) |
Philippines | |
| H. J. Axon and M. J. Nasir (1977) |
Poland | |
| Lis et al. (1986) |
| Trippke P. ( 1878) |
| Lis et al. (1986) |
| Ma et al. (2012) |
| Benjamin N. Powell (1969) |
| Chodyniecka (1967) +1 other reference |
| Góra Św. Anny 2007 |
| Duke et al. (1967) |
| Ł. Kruszewski EPMA/PXRD data +1 other reference |
| Ciesielczuk et al. (2014) |
| Kruszewski et al. (2019) |
Portugal | |
| Rui Nunes collection 2011/2012 |
| Rui Nunes collection |
| CEDOM |
| Mason B. (1979) +2 other references |
Romania | |
| Szakáll |
| Szakáll et al. (2010) | |
| Szakáll et al. (2010) |
| König et al. (2001) |
Russia | |
| Cesnokov et al. (1998) |
| Sharygin et al. (2010) |
| Gurbanov et al. (2008) |
| Sidorov (2019) |
| Kaminsky et al. (2016) |
| Active Volcanoes of Kamchatka |
| Zhitova et al. (2022) |
| Frolova et al. (2010, April) | |
| Bortnikova et al. (2017) |
| Migdisova et al. (1988) +1 other reference |
| Kokh et al. (2023) |
| Sokol et al. (2019) |
| Masaitis (1998) |
| Lavrentjeva et al. (2013) |
| Khomyakov et al. (2002) |
| Skripnik (1980) +1 other reference |
| Talovina et al. (2003) |
| Zamiatina et al. (2023) |
| Frolova et al. (2010, April) |
| maurice.strahlen.org (2004) +1 other reference | |
| Zhitova et al. (2020) |
| Vergasova et al. (1977) +1 other reference | |
| Prinz et al. (1980) |
| Prinz et al. (1980) +1 other reference |
Saudi Arabia | |
| Kraut et al. (1971) +1 other reference |
Slovakia | |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Ďuďa (1993) |
| Koděra (1986) |
| Myšľan P. 2020: Minerály z lomov ... | |
| Pauliš P. |
| Bacsó |
| Bacsó |
| Pauliš P. |
| Koděra | |
| Kaličiak |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) | |
| Duda eut al. |
| Duda et all. |
| Myšľan P. (2021) | |
| Duda et. all. |
| Pauliš |
| Duda et all. |
| Koděra (1986) | |
| Duda et all. |
| Ďuďa |
| Koděra (1986) |
South Africa | |
| Cairncross et al. (1995) +1 other reference |
| Kimura et al. (2005) |
| Floran et al. (1978) +1 other reference |
South Sudan | |
| Duke (1967) |
Spain | |
| |
| Abad +3 other references |
| Joaquim Mollfulleda |
| Mason et al. (1973) +2 other references |
| Sevillano et al. (2009) |
| |
Tajikistan | |
| Sharygin et al. (2009) |
Tunisia | |
| S Tunisia +1 other reference | |
Turkey | |
| Sarp et al. (2005) |
| Özen et al. (2013) |
UK | |
| Golley et al. (1995) |
| www.habitas.org.uk (n.d.) +1 other reference |
| Chinner et al. (1973) |
| Mittlefehldt (1998) |
Ukraine | |
| Labotka et al. (1980) |
USA | |
| Duke (1967) |
| Prinz et al. (1980) +1 other reference |
| Anthony et al. (1995) |
| Grundel (2002) | |
| Thomas (1953) +1 other reference |
| Meteoritics & Planetary Science 36 (2001) |
| Data provided by Janet Clifford |
| Galbraith (1959) +1 other reference | |
| Schrader (1915) |
| Rocks & Min. (1995) +1 other reference |
| Van Nostrand Reinholt Press: 351. +5 other references |
| Starkey et al. (1979) +1 other reference |
| Martin et al. (2020) |
| Rogers (1922) +1 other reference |
| Pemberton (1983) | |
| Cal Div of Mines & Geology "Mineral ... |
| Anderson (1936) +1 other reference | |
| Anderson (1936) +2 other references |
| M.Chorazewicz collection |
| Van Nostrand Reinholt Press: 353. +2 other references |
| Rogers (1928) +1 other reference | |
| Van Nostrand Reinholt Press: 354. +2 other references |
| Van Nostrand Reinholt Press: 354. +2 other references |
| Schaller (1905b) +1 other reference |
| Rogers (1933) +1 other reference |
| Van Nostrand Reinholt Press: 354. +2 other references |
| MarekC (2018) +1 other reference |
| Perry +1 other reference |
| Van Nostrand Reinholt Press: 354. +3 other references |
| Van Nostrand Reinholt Press: 354. +3 other references |
| Murdoch (1966) |
| Van Nostrand Reinholt Press: 381. +5 other references |
| Van Nostrand Reinholt Press: 354. +3 other references |
| Van Nostrand Reinholt Press: 438. +2 other references |
| Murdoch (1966) +1 other reference |
| Van Nostrand Reinholt Press: 318. +3 other references |
| Rogers (1912) +3 other references |
| USGS Bull 1716C +1 other reference |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| American Mineralogist +1 other reference |
| Meteoritics |
| John T. Stark (1963) | |
| in the collection of Christof Schäfer |
| Ream (1995) |
| - (2005) |
| Roadcap et al. (2005) |
| Fuchs L H (1967) +2 other references |
| Fuchs (1968) |
| Boesenberg et al. (1997) +1 other reference |
| Akihiko Okada (1980) |
| Mason B. (1979) |
| Sherwood et al. (1998) |
| Floran (1978) +1 other reference |
| Winchell (1914) |
| Cossaboom (1981) +1 other reference |
| Mészárosová (2018) |
| Duke (1967) |
| Castor et al. (2004) |
| Rocks & Minerals +1 other reference |
| - (2005) | |
| Stewart et al. (1977) +2 other references | |
| Stewart et al. (1977) +1 other reference | |
| Castor et al. (2004) |
| Noble et al. (1968) |
| CONF-910435-37 +2 other references | |
| Castor et al. (2004) |
| Walstrom (n.d.) |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Northrop et al. (1996) |
| NMGS 37th Field Conference |
| Min.Rec.: 20 (5) | |
| Northrop et al. (1996) |
| Ronald Gibbs +1 other reference | |
| Northrop et al. (1996) | |
| Noonan (1975) +1 other reference |
| Northrop et al. (1996) |
| Northrop et al. (1996) |
| Northrop et al. (1996) | |
| Isik et al. (1994) | |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| Gibbs (2013) | |
| Northrop et al. (1996) |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| Jerry Cone Collection | |
| Collected by Mike Reinke | |
| NMBGMR Open-file Report - 535 |
| D.Court collection +1 other reference | |
| Ramon DeMark and Jessie Kline |
| NMBMMR New Mexico Mineral symposium (2000) | |
| Northrop et al. (1996) | |
| Foshag (1938) +2 other references | |
| Hess et al. (1949) +3 other references |
| Bargar et al. (1986) |
| K. Starnes |
| Mineralogical Record Vol 16 Num 2 | |
| Kleck. W. D. (1970) |
| Olsen et al. (1970) +1 other reference |
| Douglas Merson collection |
| - (2005) |
| Eckel et al. (1997) | |
| Smith et al. (2000) |
| Mason et al. (1973) +1 other reference |
| PEDRAZA ROJAS et al. (2016) |
| R. Stach and J. Harkensen (1974) |
| Farrington (1915) +1 other reference |
| Floran (1978) +1 other reference |
| Duke et al. (1967) |
| www.meteorites.com.au +1 other reference |
| Nassau K. (1968) |
| Heyse (1975) +1 other reference |
| Rocks & Minerals: 68 (6) |
| Mineralogical Record: 17 (5) | |
| Thorne (n.d.) | |
| Robert Werner (2016) |
| Bullock (1981) |
| Dietrich (1990) |
| articles.adsabs.harvard.edu (n.d.) +1 other reference |
| Rye et al. (2003) | |
| Frank (1983) |
| Hawley (1934) |
| Gunnar Färber - Mineralienliste 3-2017. |
| AmMin 72:137 |
| Iddings et al. (1892) |
Vanuatu | |
| Coulon et al. (New Hebrides) |
Mars | |
| Milliken et al. (2016) +1 other reference |
| Morris et al. (2016) | |
Northwest Africa Meteorites | |
| www.cometshopnew.com (n.d.) | |
The Moon | |
| El Goresy et al. (1971) |
| Kaneko et al. (2015) |
| Geochemica et Cosmochimica Acta: 34 (1) | |
| Vol.2: Chemical Isotope Analyses +2 other references |
Quick NavTopAbout TridymiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystallographic forms Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesVarietiesCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Caspar quarry, Ettringen, Vordereifel, Mayen-Koblenz, Rhineland-Palatinate, Germany