Mallardite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Mallardite
Formula:
MnSO4 · 7H2O
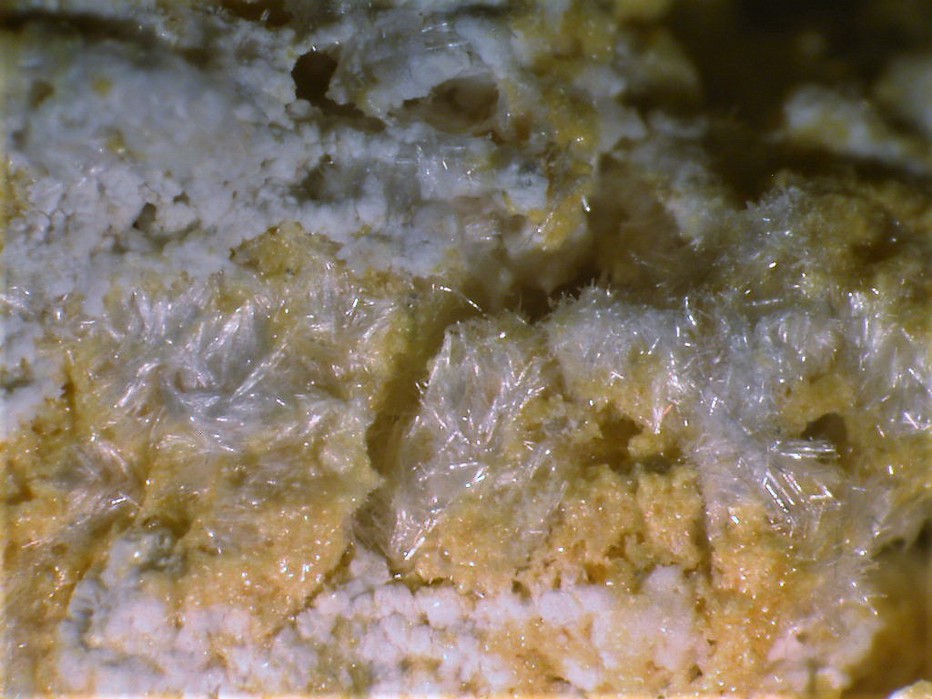
Colour:
Light rose pink; colourless in transmitted light.
Lustre:
Vitreous
Hardness:
2
Specific Gravity:
1.846
Crystal System:
Monoclinic
Member of:
Name:
Named in 1879 A. Carnot in honor of François Ernest Mallard [4 February 1833, Châteauneuf-sur-Cher, France - 6 July 1894, Paris, France], French crystallographer. Mallard was trained both as a mining engineer and as a mineralogist. In 1859, he was professor at the School of Mines (Saint Etienne) and, in 1872, professor at the School of Mines in Paris. Mallard made many contributions. Particularly, he solved important problems relating to minerals that had anomalous optical properties and discovered that minerals with low symmetry could appear to have higher symmetry due to stacking of small low-symmetry domains. This discovery led to solutions to the problems relating to pseudo-symmetry and optical effects relating to crystal clusters. Mallard wrote the important two volume, Traité de Cristallographie, in 1879 and 1884. Because of his practical nature as an engineer, Mallard's work with Henry Le Chatelier solved issues relating to gas explosions in mines. Mallard was also a field mapping geologist.
Unique Identifiers
Mindat ID:
2555
Long-form identifier:
mindat:1:1:2555:9
GUID
(UUID V4):
(UUID V4):
382ce99a-ceb1-48f6-8c5f-2aba4514a12d
IMA Classification of Mallardite
Approved, 'Grandfathered' (first described prior to 1959)
IMA Formula:
Mn(SO4) · 7H2O
First published:
1879
Classification of Mallardite
7.CB.35
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
29.6.10.5
29 : HYDRATED ACID AND NORMAL SULFATES
6 : AXO4·xH2O
29 : HYDRATED ACID AND NORMAL SULFATES
6 : AXO4·xH2O
25.9.3
25 : Sulphates
9 : Sulphates of Mn
25 : Sulphates
9 : Sulphates of Mn
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Mal | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Mallardite
Vitreous
Transparency:
Transparent, Translucent
Colour:
Light rose pink; colourless in transmitted light.
Streak:
White
Hardness:
2 on Mohs scale
Cleavage:
Distinct/Good
On {001} good; possibly also on {110}.
On {001} good; possibly also on {110}.
Density:
1.846 g/cm3 (Measured) 1.838 g/cm3 (Calculated)
Comment:
Density measured on artificial material.
Optical Data of Mallardite
Type:
Biaxial (+)
RI values:
nα = 1.462 nβ = 1.465 nγ = 1.474
Max Birefringence:
δ = 0.012

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Moderate
Dispersion:
r > v strong
Optical Extinction:
Y = b; Z ∧ c = 43°–44°.
Chemistry of Mallardite
Mindat Formula:
MnSO4 · 7H2O
Elements listed:
Crystallography of Mallardite
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P2/m
Cell Parameters:
a = 14.15 Å, b = 6.5 Å, c = 11.06 Å
β = 105.6°
β = 105.6°
Ratio:
a:b:c = 2.177 : 1 : 1.702
Unit Cell V:
979.77 ų (Calculated from Unit Cell)
Z:
4
Morphology:
Artificial crystals are tabular {001}. Fibrous masses and crusts.
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 4.92 Å | (100) |
| 5.49 Å | (72) |
| 4.88 Å | (54) |
| 3.79 Å | (42) |
| 2.758 Å | (38) |
| 3.26 Å | (36) |
| 3.13 Å | (31) |
Comments:
Jokoku mine, Japan.
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 47b : [Sulfates and sulfites] | |
| 47e : [Vanadates, chromates, manganates] | |
| 47h : [Near-surface oxidized, dehydrated minerals] | |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 55 : Anthropogenic mine minerals |
Geological Setting:
Oxidation of iron/manganese sulfides and carbonates in water saturated environments.
Type Occurrence of Mallardite
General Appearance of Type Material:
Fibrous.
Place of Conservation of Type Material:
Natural History Museum, Paris, France, number 96132.
Other Language Names for Mallardite
German:Mallardit
Spanish:Mallardita
Relationship of Mallardite to other Species
Member of:
Other Members of this group:
| Alpersite | (Mg,Cu)(SO4) · 7H2O | Mon. 2/m : P21/b |
| Bieberite | CoSO4 · 7H2O | Mon. 2/m : P2/m |
| Boothite | CuSO4 · 7H2O | Mon. 2/m : P21/b |
| Melanterite | Fe2+(H2O)6SO4 · H2O | Mon. 2/m : P21/b |
| Zincmelanterite | (Zn,Cu,Fe)SO4 · 7H2O | Mon. 2/m : P21/b |
Common Associates
Associated Minerals Based on Photo Data:
| 1 photo of Mallardite associated with Chvaleticeite | Mn(SO4) · 6H2O |
Related Minerals - Strunz-mindat Grouping
| 7.CB. | Sarvodaite | Al2(SO4)3 · 5H2O |
| 7.CB.02 | Voudourisite | CdSO4 · H2O |
| 7.CB.05 | Dwornikite | Ni(SO4) · H2O |
| 7.CB.05 | Gunningite | ZnSO4 · H2O |
| 7.CB.05 | Kieserite | MgSO4 · H2O |
| 7.CB.05 | Poitevinite | (Cu,Fe)SO4 · H2O |
| 7.CB.05 | Szmikite | MnSO4 · H2O |
| 7.CB.05 | Szomolnokite | FeSO4 · H2O |
| 7.CB.05 | Cobaltkieserite | CoSO4 · H2O |
| 7.CB.07 | Sanderite | MgSO4 · 2H2O |
| 7.CB.10 | Bonattite | CuSO4 · 3H2O |
| 7.CB.12 | Belogubite | CuZn(SO4)2 · 10H2O |
| 7.CB.15 | Aplowite | (Co,Mn,Ni)SO4 · 4H2O |
| 7.CB.15 | Boyleite | (Zn,Mg)SO4 · 4H2O |
| 7.CB.15 | Ilesite | (Mn,Zn,Fe)SO4 · 4H2O |
| 7.CB.15 | Rozenite | FeSO4 · 4H2O |
| 7.CB.15 | Starkeyite | MgSO4 · 4H2O |
| 7.CB.15 | Drobecite | CdSO4 · 4H2O |
| 7.CB.15 | Cranswickite | MgSO4 · 4H2O |
| 7.CB.20 | Chalcanthite | CuSO4 · 5H2O |
| 7.CB.20 | Jôkokuite | MnSO4 · 5H2O |
| 7.CB.20 | Pentahydrite | MgSO4 · 5H2O |
| 7.CB.20 | Siderotil | FeSO4 · 5H2O |
| 7.CB.25 | Bianchite | Zn(SO4) · 6H2O |
| 7.CB.25 | Chvaleticeite | Mn(SO4) · 6H2O |
| 7.CB.25 | Ferrohexahydrite | FeSO4 · 6H2O |
| 7.CB.25 | Hexahydrite | MgSO4 · 6H2O |
| 7.CB.25 | Moorhouseite | Co(SO4) · 6H2O |
| 7.CB.25 | Nickelhexahydrite | Ni(SO4) · 6H2O |
| 7.CB.30 | Retgersite | NiSO4 · 6H2O |
| 7.CB.35 | Bieberite | CoSO4 · 7H2O |
| 7.CB.35 | Boothite | CuSO4 · 7H2O |
| 7.CB.35 | Melanterite | Fe2+(H2O)6SO4 · H2O |
| 7.CB.35 | Zincmelanterite | (Zn,Cu,Fe)SO4 · 7H2O |
| 7.CB.35 | Alpersite | (Mg,Cu)(SO4) · 7H2O |
| 7.CB.40 | Epsomite | MgSO4 · 7H2O |
| 7.CB.40 | Goslarite | ZnSO4 · 7H2O |
| 7.CB.40 | Morenosite | NiSO4 · 7H2O |
| 7.CB.45 | Alunogen | Al2(SO4)3 · 17H2O |
| 7.CB.45 | Meta-alunogen | Al2(SO4)3 · 12H2O |
| 7.CB.50 | Aluminocoquimbite | Al2Fe2(SO4)6(H2O)12 · 6H2O |
| 7.CB.50 | Lazaridisite | 3CdSO4 · 8H2O |
| 7.CB.52 | Pararaisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.55 | Coquimbite | AlFe3(SO4)6(H2O)12 · 6H2O |
| 7.CB.55 | Paracoquimbite | Fe4(SO4)6(H2O)12 · 6H2O |
| 7.CB.55 | Rhomboclase | (H5O2)Fe3+(SO4)2 · 2H2O |
| 7.CB.55 | Raisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.57 | Caichengyunite | Fe2+3Al2(SO4)6 · 30H2O |
| 7.CB.60 | Kornelite | Fe2(SO4)3 · 7H2O |
| 7.CB.65 | Quenstedtite | Fe2(SO4)3 · 11H2O |
| 7.CB.70 | Lausenite | Fe2(SO4)3 · 5H2O |
| 7.CB.75 | Lishizhenite | ZnFe2(SO4)4 · 14H2O |
| 7.CB.75 | Römerite | Fe2+Fe3+2(SO4)4 · 14H2O |
| 7.CB.80 | Ransomite | CuFe2(SO4)4 · 6H2O |
| 7.CB.85 | Apjohnite | Mn2+Al2(SO4)4 · 22H2O |
| 7.CB.85 | Bílinite | Fe2+Fe3+2(SO4)4 · 22H2O |
| 7.CB.85 | Dietrichite | (Zn,Fe2+,Mn2+)Al2(SO4)4 · 22H2O |
| 7.CB.85 | Halotrichite | FeAl2(SO4)4 · 22H2O |
| 7.CB.85 | Pickeringite | MgAl2(SO4)4 · 22H2O |
| 7.CB.85 | Redingtonite | (Fe2+,Mg,Ni)(Cr,Al)2(SO4)4 · 22H2O |
| 7.CB.85 | Wupatkiite | (Co,Mg,Ni)Al2(SO4)4 · 22H2O |
| 7.CB.90 | Meridianiite | MgSO4 · 11H2O |
Other Information
Thermal Behaviour:
Heated in a closed tube, it gives water in abundance. Gradually calcined, it releases sulfuric acid vapors and leaves a reddish-brown residue.
Notes:
Water-soluble; quickly dehydrates at room temperature.
Special Storage/
Display Requirements:
Display Requirements:
Quickly dehydrates at room temperature.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Mallardite
mindat.org URL:
https://www.mindat.org/min-2555.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Mallardite
Localities for Mallardite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Australia | |
| Lawrence et al. (1993) |
Czech Republic | |
| Lapis 2002 (7/8) |
| Pašava +7 other references |
France | |
| Wittern et al. (Cologne) |
Italy | |
| Russo et al. (2004) |
| Exel (1987) |
Japan | |
| Encyclopedia of Mins. +1 other reference |
Peru | |
| Smuda +4 other references |
Russia | |
| Vergasova et al. (2007) |
Spain | |
| Garcia (1996) |
USA | |
| Carnein et al. (2005) |
| Northrop et al. (1996) |
| Palache et al. (1951) | |
| Northrop et al. (1996) |
| North (2010) |
| Bullock (1981) |
Quick NavTopAbout MallarditeUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography X-Ray Powder DiffractionGeological EnvironmentType Occurrence Other LanguagesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List

 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Chvaletice, Pardubice District, Pardubice Region, Czech Republic