Hydrohonessite
A valid IMA mineral species
This page is currently not sponsored. Click here to sponsor this page.
About Hydrohonessite
Formula:
(Ni1-xFe3+x)(OH)2(SO4)x/2 · nH2O
( x > 0.5, n > 3x/2)
Colour:
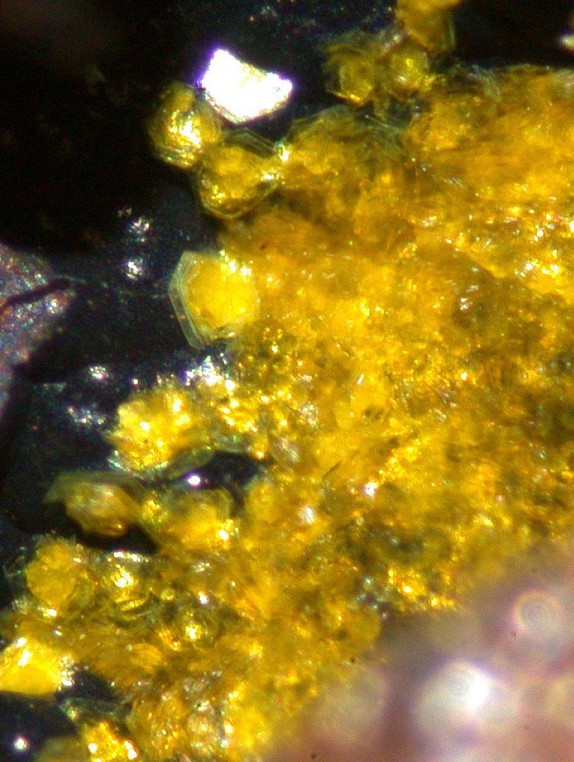
Bright yellow
Lustre:
Adamantine
Specific Gravity:
2.96 (Calculated)
Crystal System:
Hexagonal
Member of:
Name:
For its relationship (hydrated form) to Honessite.
May convert readily into honessite, depending on humidity and temperature.
Appears to be stable between pH 6 and 7.
Appears to be stable between pH 6 and 7.
Unique Identifiers
Mindat ID:
1978
Long-form identifier:
mindat:1:1:1978:7
GUID
(UUID V4):
(UUID V4):
c8ac3497-db12-4ddb-a141-d0355c9a8449
IMA Classification of Hydrohonessite
Approved
IMA Formula:
(Ni1-xFe3+x)(SO4)x/2(OH)2 · nH2O (x < 0.5, n > 3x/2)
First published:
1981
Classification of Hydrohonessite
7.DD.35
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
D : Sulfates (selenates, etc.) with additional anions, with H2O
D : With only medium-sized cations; sheets of edge-sharing octahedra
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
D : Sulfates (selenates, etc.) with additional anions, with H2O
D : With only medium-sized cations; sheets of edge-sharing octahedra
31.10.7.1
31 : HYDRATED SULFATES CONTAINING HYDROXYL OR HALOGEN
10 : Miscellaneous
31 : HYDRATED SULFATES CONTAINING HYDROXYL OR HALOGEN
10 : Miscellaneous
25.12.12
25 : Sulphates
12 : Sulphates of Co and Ni
25 : Sulphates
12 : Sulphates of Co and Ni
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Hhon | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Hhon | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Physical Properties of Hydrohonessite
Adamantine
Transparency:
Transparent, Translucent
Colour:
Bright yellow
Hardness Data:
Could not be measured
Density:
2.96 g/cm3 (Calculated)
Comment:
Could not be measured due to fine grain of the material
Optical Data of Hydrohonessite
Type:
Uniaxial (-)
RI values:
nω = 1.630 nε = 1.590
Max Birefringence:
δ = 0.040

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Moderate
Chemistry of Hydrohonessite
Mindat Formula:
(Ni1-xFe3+x)(OH)2(SO4)x/2 · nH2O
( x > 0.5, n > 3x/2)
( x > 0.5, n > 3x/2)
Crystallography of Hydrohonessite
Crystal System:
Hexagonal
Cell Parameters:
a = 3.09 Å, c = 10.8 Å
Ratio:
a:c = 1 : 3.495
Unit Cell V:
89.30 ų (Calculated from Unit Cell)
Morphology:
hexagonal flakes, to 10 µm.
Comment:
Point Group: n.d.; Space Group: n.d.;Z = n.d.
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 11.0 Å | (10) |
| 5.56 Å | (5) |
| 3.68 Å | (4) |
| 2.709 Å | (3) |
| 2.595 Å | (2) |
| 2.394 Å | (2) |
| 2.152 Å | (1) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| 47b : [Sulfates and sulfites] |
Type Occurrence of Hydrohonessite
General Appearance of Type Material:
Thin surface encrustation of tiny hexagonal crystals on botryoidal quartz and magnesite in a fracture in supergene Ni-Fe sulphides.
Place of Conservation of Type Material:
Western Australian Museum, Perth, Australia, M.77a.1991, M.77b.1991; National Museum of Natural History, Washington, D.C., USA, 150420.
Associated Minerals at Type Locality:
Reference:
Nickel, E.H., Wildman, J.E. (1981) Hydrohonessite- a new hydrated Ni-Fe hydroxy-sulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group. Mineralogical Magazine: 44: 333-337.
Synonyms of Hydrohonessite
Other Language Names for Hydrohonessite
German:Hydrohonessit
Spanish:Hydrohonessita
Relationship of Hydrohonessite to other Species
Member of:
Other Members of this group:
| Carrboydite | (Ni1-xAlx)(SO4)x/2(OH)2 · nH2O | Hex. |
| Glaucocerinite | (Zn1-xAlx)(OH)2(SO4)x/2 · nH2O | Hex. |
| Mountkeithite | [(Mg1-xFe3+x)(OH)2][SO4]x/2 · nH2O | Hex. |
| Zincaluminite | Zn6Al6(SO4)2(OH)16 · 5H2O |
Common Associates
Associated Minerals Based on Photo Data:
| 11 photos of Hydrohonessite associated with Gaspéite | NiCO3 |
| 5 photos of Hydrohonessite associated with Gillardite | Cu3Ni(OH)6Cl2 |
| 4 photos of Hydrohonessite associated with Gypsum | CaSO4 · 2H2O |
| 2 photos of Hydrohonessite associated with Paratacamite | Cu3(Cu,Zn)(OH)6Cl2 |
| 1 photo of Hydrohonessite associated with Quartz | SiO2 |
| 1 photo of Hydrohonessite associated with Tremolite | ◻Ca2Mg5(Si8O22)(OH)2 |
Related Minerals - Strunz-mindat Grouping
| 7.DD. | Asagiite | NiCu4(SO4)2(OH)6 · 6H2O |
| 7.DD.05 | Felsőbányaite | Al4(SO4)(OH)10 · 4H2O |
| 7.DD.07 | Llantenesite | Cu6Al[SeO4](OH)12Cl · 3H2O |
| 7.DD.10 | Langite | Cu4(SO4)(OH)6 · 2H2O |
| 7.DD.10 | Posnjakite | Cu4(SO4)(OH)6 · H2O |
| 7.DD.10 | Wroewolfeite | Cu4(SO4)(OH)6 · 2H2O |
| 7.DD.10 | Gobelinite | CoCu4(SO4)2(OH)6 · 6H2O |
| 7.DD.10 | Fehrite | MgCu4(SO4)2(OH)6 · 6H2O |
| 7.DD.15 | Spangolite | Cu6Al(SO4)(OH)12Cl · 3H2O |
| 7.DD.15 | Kobyashevite | Cu5(SO4)2(OH)6 · 4H2O |
| 7.DD.15 | Unnamed (Dimorph of Devilline) | CaCu4(SO4)2(OH)6 · 3H2O |
| 7.DD.20 | Ktenasite | ZnCu4(SO4)2(OH)6 · 6H2O |
| 7.DD.25 | Christelite | Cu2Zn3(SO4)2(OH)6 · 4H2O |
| 7.DD.30 | Campigliaite | Mn2+Cu4(SO4)2(OH)6 · 4H2O |
| 7.DD.30 | Devilline | CaCu4(SO4)2(OH)6 · 3H2O |
| 7.DD.30 | Orthoserpierite | Ca(Cu,Zn)4(SO4)2(OH)6 · 3H2O |
| 7.DD.30 | Serpierite | Ca(Cu,Zn)4(SO4)2(OH)6 · 3H2O |
| 7.DD.30 | Niedermayrite | CdCu4(SO4)2(OH)6 · 4H2O |
| 7.DD.30 | Edwardsite | Cu3Cd2(SO4)2(OH)6 · 4H2O |
| 7.DD.35 | Carrboydite | (Ni1-xAlx)(SO4)x/2(OH)2 · nH2O |
| 7.DD.35 | Glaucocerinite | (Zn1-xAlx)(OH)2(SO4)x/2 · nH2O |
| 7.DD.35 | Honessite | (Ni1-xFe3+x)(OH)2[SO4]x/2 · nH2O |
| 7.DD.35 | Motukoreaite | Mg6Al3(OH)18[Na(H2O)6][SO4]2 · 6H2O |
| 7.DD.35 | Mountkeithite | [(Mg1-xFe3+x)(OH)2][SO4]x/2 · nH2O |
| 7.DD.35 | Shigaite | Mn6Al3(OH)18[Na(H2O)6](SO4)2 · 6H2O |
| 7.DD.35 | Wermlandite | Mg7Al2(OH)18[Ca(H2O)6][SO4]2 · 6H2O |
| 7.DD.35 | Woodwardite | Cu1-xAlx(OH)2(SO4)x/2 · nH2O |
| 7.DD.35 | Zincaluminite | Zn6Al6(SO4)2(OH)16 · 5H2O |
| 7.DD.35 | Hydrowoodwardite | (Cu1-xAlx)(OH)2[SO4]x/2 · nH2O |
| 7.DD.35 | Zincowoodwardite | Zn1-xAlx(OH)2[SO4]x/2 · nH2O |
| 7.DD.35 | Natroglaucocerinite | Zn6Al3(OH)18[Na(H2O)6](SO4)2 · 6H2O |
| 7.DD.35 | Nikischerite | Fe2+6Al3(OH)18[Na(H2O)6](SO4)2 · 6H2O |
| 7.DD.40 | Isselite | Cu6(SO4)(OH)10 · 5H2O |
| 7.DD.40 | Lawsonbauerite | (Mn2+,Mg)9Zn4(SO4)2(OH)22 · 8H2O |
| 7.DD.40 | Torreyite | (Mg,Mn2+)7◻2Mn2+2Zn4(SO4)2(OH)22 · 8H2O |
| 7.DD.45 | Mooreite | Mg9◻2Mn2Zn4(SO4)2(OH)26 · 8H2O |
| 7.DD.45 | Hodgesmithite | (Cu,Zn)6Zn(SO4)2(OH)10 · 3H2O |
| 7.DD.47 | Lahnsteinite | Zn4(SO4)(OH)6 · 3H2O |
| 7.DD.50 | Namuwite | Zn4(SO4)(OH)6 · 4H2O |
| 7.DD.50 | Minohlite | (Cu,Zn)7(SO4)2(OH)10 · 8H2O |
| 7.DD.52 | Lauraniite | Cu6Cd2(SO4)2(OH)12 · 5H2O |
| 7.DD.55 | Bechererite | Zn7Cu(OH)13[(SiO(OH)3(SO4)] |
| 7.DD.60 | Ramsbeckite | (Cu,Zn)15(SO4)4(OH)22 · 6H2O |
| 7.DD.65 | Vonbezingite | Ca6Cu3(SO4)3(OH)12 · 2H2O |
| 7.DD.70 | Redgillite | Cu6(SO4)(OH)10 · H2O |
| 7.DD.75 | Chalcoalumite | CuAl4(SO4)(OH)12 · 3H2O |
| 7.DD.75 | Nickelalumite | NiAl4(SO4)(OH)12(H2O)3 |
| 7.DD.75 | Kyrgyzstanite | ZnAl4(SO4)(OH)12 · 3H2O |
| 7.DD.80 | Guarinoite | Zn6(SO4)(OH)10 · 5H2O |
| 7.DD.80 | Schulenbergite | (Cu,Zn)7(SO4)2(OH)10 · 3H2O |
| 7.DD.80 | Thérèsemagnanite | NaCo4(SO4)(OH)6Cl · 6H2O |
| 7.DD.80 | UM1992-30-SO:CCuHZn | (Zn,Cu)7(SO4,CO3)2(OH)10 · 3H2O |
| 7.DD.85 | Montetrisaite | Cu6(SO4)(OH)10 · 2H2O |
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Hydrohonessite
mindat.org URL:
https://www.mindat.org/min-1978.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Hydrohonessite
Reference List:
Nickel, Ernest H., Wildman, John E. (1981) Hydrohonessite—a new hydrated Ni-Fe hydroxy-sulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group. Mineralogical Magazine, 44 (335) 333-337 doi:10.1180/minmag.1981.044.335.14
Bish, D. L., Livingstone, A. (1981) The crystal chemistry and paragenesis of honessite and hydrohonessite: the sulphate analogues of reevesite. Mineralogical Magazine, 44 (335) 339-343 doi:10.1180/minmag.1981.044.335.15
Nickel, Ernest H., Wildman, John E. (1981) Hydrohonessite—a new hydrated Ni-Fe hydroxy-sulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group. Mineralogical Magazine, 44 (335) 333-337 doi:10.1180/minmag.1981.044.335.14
Livingstone, A. (1990) Copper–aluminium analogues of hydrohonessite and honessite, and woodwardite relationships. Mineralogical Magazine, 54 (377) 649-653 doi:10.1180/minmag.1990.054.377.20
Frost, Ray L., Weier, Matt L., Kloprogge, J. Theo (2003) Raman spectroscopy of some natural hydrotalcites with sulphate and carbonate in the interlayer. Journal of Raman Spectroscopy, 34 (10). 760-768 doi:10.1002/jrs.1050
Localities for Hydrohonessite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Australia | |
| R Bottrill |
| Nickel et al. (1981) |
| Nickel et al. (1993) +1 other reference |
| Nickel et al. (1981) +1 other reference |
Greece | |
| Branko Rieck collection |
Italy | |
| Adorni F. (1997) |
| Rivista Mineralogica Italiana (3) |
| Bonifazi (2020) +3 other references |
Japan | |
| Yamada (2004) |
Russia | |
| Bortnikova et al. (2017) |
Spain | |
| Joan Abella i Creus (Joanabellacreus@gmail.com) |
UK | |
| Day (1999) |
| Bish et al. (1981) +2 other references |
USA | |
| Williams et al. (1995) |
| Williams (1995) +1 other reference | |
| Rocks & Minerals Vol. 70 |
| Goldstein (2006) |
Quick NavTopAbout HydrohonessiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography X-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List




 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



132 North Ni Mine, Widgiemooltha, Coolgardie Shire, Western Australia, Australia