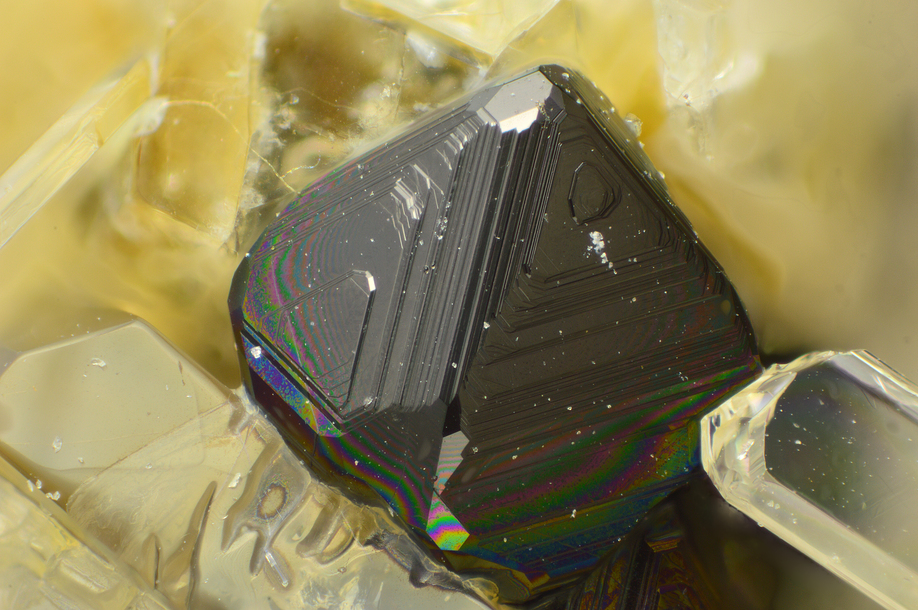
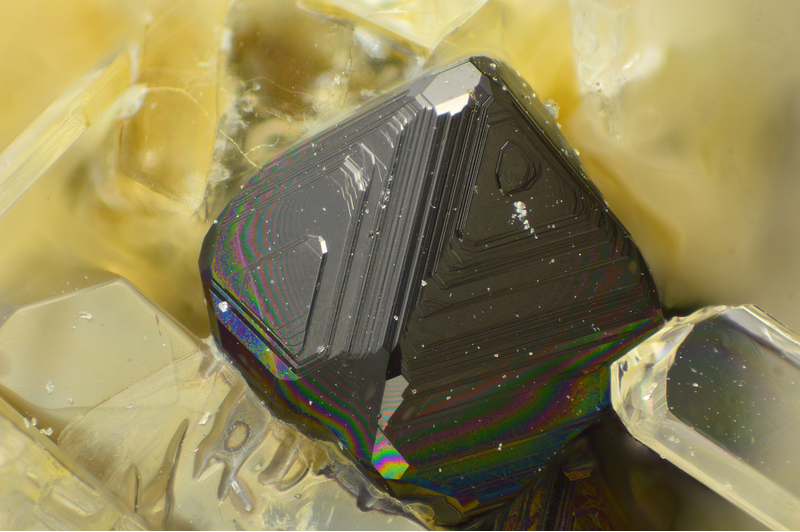
Magnetite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Magnetite
Formula:
Fe2+Fe3+2O4
As a Commodity:
Colour:
Greyish black or iron black
Lustre:
Metallic, Sub-Metallic
Hardness:
5½ - 6½
Specific Gravity:
5.175
Crystal System:
Isometric
Member of:
Name:
Originally called lodestone as early as 1548 and by other names. Named in 1845 by Wilhelm Karl von Haidinger for the locality at Magnesia, Greece (site for lodestone).
Jacobsite-Magnetite Series. Magnesioferrite-Magnetite Series.
Magnetite is an important, strongly magnetic iron ore, along with hematite.
Nanoinclusions of magnetite crystals cause the iridescence of Rainbow obsidian (Nadin, 2007). Extremely thin layers of 200-nm octahedral crystals of magnetite give some basalt surfaces an iridescent sheen (Nadin, 2007).
May precipitate from supersaturated volcanic gas due to its cooling as shown in an experiment by Africano et al. (2002) (actually a predominant precipitate from ca. 850 down to 650oC in this particular experiment).
 Visit gemdat.org for gemological information about Magnetite.
Visit gemdat.org for gemological information about Magnetite.
Magnetite is an important, strongly magnetic iron ore, along with hematite.
Nanoinclusions of magnetite crystals cause the iridescence of Rainbow obsidian (Nadin, 2007). Extremely thin layers of 200-nm octahedral crystals of magnetite give some basalt surfaces an iridescent sheen (Nadin, 2007).
May precipitate from supersaturated volcanic gas due to its cooling as shown in an experiment by Africano et al. (2002) (actually a predominant precipitate from ca. 850 down to 650oC in this particular experiment).
 Visit gemdat.org for gemological information about Magnetite.
Visit gemdat.org for gemological information about Magnetite.Unique Identifiers
Mindat ID:
2538
Long-form identifier:
mindat:1:1:2538:2
GUID
(UUID V4):
(UUID V4):
638fda79-4914-4fc0-9169-3c497a942ec8
IMA Classification of Magnetite
Approved, 'Grandfathered' (first described prior to 1959)
Classification of Magnetite
4.BB.05
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
B : Metal: Oxygen = 3:4 and similar
B : With only medium-sized cations
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
B : Metal: Oxygen = 3:4 and similar
B : With only medium-sized cations
7.2.2.3
7 : MULTIPLE OXIDES
2 : AB2X4
7 : MULTIPLE OXIDES
2 : AB2X4
7.20.2
7 : Oxides and Hydroxides
20 : Oxides of Fe
7 : Oxides and Hydroxides
20 : Oxides of Fe
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Mag | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Mag | Kretz (1983) | Kretz, R. (1983) Symbols of rock-forming minerals. American Mineralogist, 68, 277–279. |
| Mag | Siivolam & Schmid (2007) | Siivolam, J. and Schmid, R. (2007) Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: List of mineral abbreviations. Web-version 01.02.07. IUGS Commission on the Systematics in Petrology. download |
| Mag | Whitney & Evans (2010) | Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 doi:10.2138/am.2010.3371 |
| Mgt | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
| Mag | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Physical Properties of Magnetite
Metallic, Sub-Metallic
Transparency:
Opaque
Colour:
Greyish black or iron black
Streak:
Black
Hardness:
5½ - 6½ on Mohs scale
Hardness:
VHN100=681 - 792 kg/mm2 - Vickers
Tenacity:
Brittle
Parting:
On {111}, especially good. Also reported as parting planes: {001}, {011}, {138}.
Fracture:
Irregular/Uneven
Density:
5.175 g/cm3 (Measured) 5.2 g/cm3 (Calculated)
Optical Data of Magnetite
Type:
Isotropic
RI values:
n = 2.42
Birefringence:
Isotropic minerals have no birefringence
Surface Relief:
Very High
Reflectivity:
| Wavelength | R |
|---|---|
| 400nm | 22.3% |
| 420nm | 21.8% |
| 440nm | 21.3% |
| 460nm | 20.8% |
| 480nm | 20.5% |
| 500nm | 20.3% |
| 520nm | 20.3% |
| 540nm | 20.4% |
| 560nm | 20.5% |
| 580nm | 20.6% |
| 600nm | 20.6% |
| 620nm | 20.7% |
| 640nm | 20.8% |
| 660nm | 20.9% |
| 680nm | 21.0% |
| 700nm | 21.2% |
Graph shows reflectance levels at different wavelengths (in nm). Top of box is 100%. Peak reflectance is 22.3%.
Colour in reflected light:
Grey with brownish tint
Internal Reflections:
None
Pleochroism:
Non-pleochroic
Comments:
Twin lamellae and zonal growth pattern exhibited in polished section by magnetite at times.
Chemistry of Magnetite
Mindat Formula:
Fe2+Fe3+2O4
Elements listed:
Common Impurities:
Mg,Zn,Mn,Ni,Cr,Ti,V,Al
Crystallography of Magnetite
Crystal System:
Isometric
Class (H-M):
m3m (4/m 3 2/m) - Hexoctahedral
Space Group:
Fd3m
Cell Parameters:
a = 8.396 Å
Unit Cell V:
591.86 ų (Calculated from Unit Cell)
Z:
8
Morphology:
Crystals usually octahedral, sometimes dodecahedral, striated on {011} parallel [011]; less frequently with modifying {001} or {hhl}. Cubic (Balmat, NY), rare. Skeletonized microcrystals found in igneous rocks. Massive, granular, coarse to fine.
Twinning:
Common on {111}, with the same face as the composition face. Twins flattened parallel to {111} (common spinel law twins), or as lamellar twins, producing striae on {111}. Twin gliding, with K1{111}, K2{111}.
Crystallographic forms of Magnetite
Crystal Atlas:
Image Loading
Click on an icon to view
3d models and HTML5 code kindly provided by
www.smorf.nl.
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0000945 | Magnetite | Wechsler B A, Lindsley D H, Prewitt C T (1984) Crystal structure and cation distribution in titanomagnetites (Fe3-xTixO4) American Mineralogist 69 754-770 |  | 1984 | synthetic | 0 | 293 |
| 0002400 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 0 | 293 | |
| 0002401 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 1.4 | 293 | |
| 0002402 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 2.3 | 293 | |
| 0002403 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 3.4 | 293 | |
| 0002404 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 6.6 | 293 | |
| 0002405 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 8.3 | 293 | |
| 0002406 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 11.3 | 293 | |
| 0002407 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 12.5 | 293 | |
| 0002408 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 13.5 | 293 | |
| 0002409 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 15.7 | 293 | |
| 0002410 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 17.5 | 293 | |
| 0002411 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 19.4 | 293 | |
| 0002412 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 21.8 | 293 | |
| 0002413 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 24.2 | 293 | |
| 0002414 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 26.9 | 293 | |
| 0002415 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure American Mineralogist 85 514-523 |  | 2000 | 30.3 | 293 | |
| 0002416 | Magnetite | Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure Sample at 40 GPa, CaTi2O4 structure type American Mineralogist 85 514-523 |  | 2000 | 0 | 293 | |
| 0002762 | Magnetite | Fjellvag H, Hauback B C, Vogt T, Stolen S (2002) Monoclinic nearly stoichiometric wustite at low temperatures American Mineralogist 87 347-349 |  | 2002 | 0 | 10 | |
| 0002763 | Magnetite | Fjellvag H, Hauback B C, Vogt T, Stolen S (2002) Monoclinic nearly stoichiometric wustite at low temperatures American Mineralogist 87 347-349 |  | 2002 | 0 | 8 | |
| 0004815 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004816 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004817 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004818 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004819 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004820 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0004821 | Magnetite | Bosi F, Halenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvospinel series American Mineralogist 94 181-189 |  | 2009 | synthetic | 0 | 293 |
| 0005178 | Magnetite | Stout M Z, Bayliss P (1980) Crystal structure of two ferrian ulvospinels from British Columbia The Canadian Mineralogist 18 339-341 |  | 1980 | Aiyansh basalt lava flow, Nass River valley, British Columbia, Canada | 0 | 293 |
| 0005179 | Magnetite | Stout M Z, Bayliss P (1980) Crystal structure of two ferrian ulvospinels from British Columbia The Canadian Mineralogist 18 339-341 |  | 1980 | Itcha Mountain hawaiite lava flow, central British Columbia, Canada | 0 | 293 |
| 0007394 | Magnetite | Finger L W, Hazen R M, Hofmeister A M (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels Physics and Chemistry of Minerals 13 215-220 | 1986 | 0.0001 | 293 | ||
| 0007395 | Magnetite | Finger L W, Hazen R M, Hofmeister A M (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels Physics and Chemistry of Minerals 13 215-220 | 1986 | 1.3 | 293 | ||
| 0007396 | Magnetite | Finger L W, Hazen R M, Hofmeister A M (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels Physics and Chemistry of Minerals 13 215-220 | 1986 | 2.6 | 293 | ||
| 0007397 | Magnetite | Finger L W, Hazen R M, Hofmeister A M (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels Physics and Chemistry of Minerals 13 215-220 | 1986 | 3.9 | 293 | ||
| 0007398 | Magnetite | Finger L W, Hazen R M, Hofmeister A M (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels Physics and Chemistry of Minerals 13 215-220 | 1986 | 4.5 | 293 | ||
| 0007418 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 0.0001 | 293 | ||
| 0007419 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 0.63 | 293 | ||
| 0007420 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 1.55 | 293 | ||
| 0007421 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 2.09 | 293 | ||
| 0007422 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 2.76 | 293 | ||
| 0007423 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 3.67 | 293 | ||
| 0007424 | Magnetite | Nakagiri N, Manghnani M H, Ming L C, Kimura S (1986) Crystal structure of magnetite under pressure Physics and Chemistry of Minerals 13 238-244 | 1986 | 4.44 | 293 | ||
| 0007766 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007771 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007776 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007781 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007824 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007829 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007834 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0007847 | Magnetite | O'Neill H St C, Dollase W A (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4 Physics and Chemistry of Minerals 20 541-555 | 1994 | 0 | 293 | ||
| 0009109 | Magnetite | Gatta G D, Kantor I, Ballaran T B, Dubrovinsky L, McCammon C (2007) Effect of non-hydrostatic conditions on the elastic behaviour of magnetite: an in situ single-crystal X-ray diffraction study Physics and Chemistry of Minerals 34 627-635 | 2007 | synthetic | 0.0001 | 293 | |
| 0009110 | Magnetite | Gatta G D, Kantor I, Ballaran T B, Dubrovinsky L, McCammon C (2007) Effect of non-hydrostatic conditions on the elastic behaviour of magnetite: an in situ single-crystal X-ray diffraction study Physics and Chemistry of Minerals 34 627-635 | 2007 | synthetic | 4.99 | 293 | |
| 0009111 | Magnetite | Gatta G D, Kantor I, Ballaran T B, Dubrovinsky L, McCammon C (2007) Effect of non-hydrostatic conditions on the elastic behaviour of magnetite: an in situ single-crystal X-ray diffraction study Physics and Chemistry of Minerals 34 627-635 | 2007 | synthetic | 9.21 | 293 | |
| 0009740 | Magnetite | Fleet M E (1981) The structure of magnetite Acta Crystallographica B37 917-920 |  | 1981 | 0 | 293 | |
| 0009994 | Magnetite | Fleet M E (1984) The structure of magnetite: two annealed natural magnetites, Fe3.005O4 and Fe2.96Mg0.04O4 Acta Crystallographica C40 1491-1493 |  | 1984 | 0 | 293 | |
| 0009995 | Magnetite | Fleet M E (1984) The structure of magnetite: two annealed natural magnetites, Fe3.005O4 and Fe2.96Mg0.04O4 Acta Crystallographica C40 1491-1493 |  | 1984 | 0 | 293 | |
| 0017295 | Magnetite | Montoro V (1938) Miscibilita fra gli ossidi salini di ferro e di manganese _cod_database_code 1010369 Gazzetta Chimica Italiana 68 728-733 | 1938 | 0 | 293 | ||
| 0020586 | Magnetite | Fleet M E (1986) The structure of magnetite: Symmetry of cubic spinels Journal of Solid State Chemistry 62 75-82 | 1986 | Natural, not provided | 0 | 293 | |
| 0020587 | Magnetite | Fleet M E (1986) The structure of magnetite: Symmetry of cubic spinels Journal of Solid State Chemistry 62 75-82 | 1986 | Natural, not provided | 0 | 293 | |
| 0020588 | Magnetite | Fleet M E (1986) The structure of magnetite: Symmetry of cubic spinels Journal of Solid State Chemistry 62 75-82 | 1986 | Natural, not provided | 0 | 293 | |
| 0020589 | Magnetite | Fleet M E (1986) The structure of magnetite: Symmetry of cubic spinels Journal of Solid State Chemistry 62 75-82 | 1986 | Natural, not provided | 0 | 293 | |
| 0020590 | Magnetite | Fleet M E (1986) The structure of magnetite: Symmetry of cubic spinels Journal of Solid State Chemistry 62 75-82 | 1986 | Natural, not provided | 0 | 293 | |
| 0013895 | Magnetite | Fjellvag H, Gronvold F, Stolen S, Hauback B C (1996) On the crystallographic and magnetic structures of nearly stoichiometric iron monoxide Journal of Solid State Chemistry 124 52-57 | 1996 | synthetic | 0 | 293 | |
| 0013896 | Magnetite | Fjellvag H, Gronvold F, Stolen S, Hauback B C (1996) On the crystallographic and magnetic structures of nearly stoichiometric iron monoxide Journal of Solid State Chemistry 124 52-57 | 1996 | synthetic | 0 | 293 | |
| 0017938 | Magnetite | Bragg W (1915) The Structure of Magnetite and the Spinels _cod_database_code 1011032 Nature (London) 95 561-561 | 1915 | 0 | 293 | ||
| 0017981 | Magnetite | Claassen A (1926) The scattering power of oxygen and iron for X-Rays _cod_database_code 1011084 Proceedings of the Physical Society of London 38 482-487 | 1926 | 0 | 293 |
CIF Raw Data - click here to close
Epitaxial Relationships of Magnetite
Epitaxial Minerals:
| Rutile | TiO2 |
| Pyrophanite | Mn2+TiO3 |
| Pseudobrookite | Fe2TiO5 |
| Muscovite | KAl2(AlSi3O10)(OH)2 |
| Ilmenite | Fe2+TiO3 |
| Hematite | Fe2O3 |
| Fayalite-Forsterite Series |
Epitaxy Comments:
Hematite overgrowths on, and inclusions in, magnetite; ilmenite inclusions, rutile overgrowths, chlorite group overgrowths, pyrophanite inclusions; magnetite on hematite; inclusions in muscovite; inclusions in hematite; inclusions in ilmenite; magnetite overgrowths on olivine.
Pseudobrookite on magnetite with pseudobrookite {100}[001] parallel to magnetite {111}[110].
Pseudobrookite on magnetite with pseudobrookite {100}[001] parallel to magnetite {111}[110].
X-Ray Powder Diffraction
Image Loading
Radiation - Copper Kα
Data courtesy of RRUFF project at University of Arizona, used with permission.
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 4.852 Å | (8) |
| 2.967 Å | (30) |
| 2.532 Å | (100) |
| 2.4243 Å | (8) |
| 2.0993 Å | (20) |
| 1.7146 Å | (10) |
| 1.6158 Å | (30) |
| 1.4845 Å | (40) |
| 1.4192 Å | (2) |
| 1.3277 Å | (4) |
| 1.2807 Å | (10) |
| 1.2659 Å | (4) |
| 1.2119 Å | (2) |
| 1.1221 Å | (4) |
| 1.0930 Å | (12) |
| 1.0496 Å | (6) |
| 0.9896 Å | (2) |
| 0.9695 Å | (6) |
| 0.9632 Å | (4) |
| 0.9388 Å | (4) |
| 0.8952 Å | (2) |
| 0.8802 Å | (6) |
| 0.8569 Å | (8) |
| 0.8233 Å | (4) |
| 0.8117 Å | (6) |
Comments:
ICDD 19-629
Geological Environment
Paragenetic Mode(s):
Geological Setting:
Common igneous accessory mineral. In sedimentary banded iron formations.
Synonyms of Magnetite
Other Language Names for Magnetite
Arabic:ماغنتيت
Catalan:Magnetita
Croatian:Magnetit
Czech:Magnetit
Dutch:Magnetiet
Estonian:Magnetiit
Finnish:Magnetiitti
Galician:Magnetita
German:Magnetit
Eisenmohr
Eisenmulm
Eisenoxydoxydul
Ferroferrit
Hammerschlag
Magneteisenerz
Magneteisenstein
Magnetischer Eisenstein
Morpholit
Siegelstein
Syderit
Eisenmohr
Eisenmulm
Eisenoxydoxydul
Ferroferrit
Hammerschlag
Magneteisenerz
Magneteisenstein
Magnetischer Eisenstein
Morpholit
Siegelstein
Syderit
Greek:Μαγνητίτης
Hebrew:מגנטיט
Hungarian:Magnetit
Japanese:磁鉄鉱
Lithuanian:Magnetitas
Norwegian:Magnetitt
Magnetjernstein
Magnetjernstein
Polish:Magnetyt
Portuguese:Magnetite
Romanian:Magnetit
Russian:Магнетит
Serbian:Магнетит
Simplified Chinese:磁铁矿
Slovak:Magnetit
Swedish:Magnetit
Magnetjernmalm
Svertmalm
Magnetjernmalm
Svertmalm
Traditional Chinese:磁鐵礦
Turkish:Manyetit
Ukrainian:Магнетит
Varieties of Magnetite
| Aluminous Magnetite | Al-rich variety of magnetite |
| Chrommagnetite | A member of Chromite-Magnetite Series - a chromian variety of Magnetite with Cr3+ contents 0,50-1 apfu. |
| Hydromagnetite | A hydrated Magnetite. |
| Ishkulite | A Cr-bearing variety of magnetite with Cr3+ contents of 0.10-0.50 apfu. A member of the Chromite-Magnetite Series. |
| Lodestone | A variety of magnetite that is a natural magnet (magnetized). |
| Magnomagnetite | Intermediate members of Magnetite-Magnesioferrite series with Fe2+>Mg. |
| Manganmagnetite | A variety of magnetite containing Mn 2+ substituting for Fe 2+. |
| Mg-Titanomagnetite | Titanium- and magnesium-rich magnetite. |
| Mushketovite | The name given for magnetite pseudomorphs after hematite. For pseudomorphs of hematite after magnetite, see "martite". |
| Nickel-bearing Magnetite | |
| Silfbergite (of Niggli) | A manganoan variety of magnetite. Contains Mn 2+ substituting for Fe 2+. |
| Tin-bearing Magnetite | |
| Titanium-bearing Magnetite | A titanium-bearing variety of magnetite. |
| Vanadium and Titanium-bearing Magnetite | May also be formulated as vanadiferous titanomagnetite. |
| Vanadium-bearing Magnetite | A V-bearing variety of magnetite. V content reported up to 4.84% (India). |
| Vanodiochrome spinel | A vanadian chromian Magnetite variety. Using the name "Vanadiochrome spinel", Mathiesen (1970) describes a magnetite containing large amounts of vanadium (16,98% V2O3) and chrome (Cr2O3), occuring in a small area with felsic rock cut by veins filled with ... |
| Zinc-bearing Magnetite | Intermediate members of the Magnetite-Franklinite series with Fe2+>Zn. The ZnO content may reach 12.9 mass%, equalling 0.35 apfu. |
Relationship of Magnetite to other Species
Member of:
Other Members of this group:
| Chromite | Fe2+Cr3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Cochromite | CoCr2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Coulsonite | Fe2+V3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Cuprospinel | Cu2+Fe3+2O4 | Iso. m3m (4/m 3 2/m) |
| Dellagiustaite | V2+Al2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Deltalumite | (Al0.67◻0.33)Al2O4 | Tet. 4 2m : P4m2 |
| Franklinite | Zn2+Fe3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Gahnite | ZnAl2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Galaxite | Mn2+Al2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Guite | Co2+Co3+2O4 | Iso. |
| Hausmannite | Mn2+Mn3+2O4 | Tet. 4/mmm (4/m 2/m 2/m) : I41/amd |
| Hercynite | Fe2+Al2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Hetaerolite | ZnMn2O4 | Tet. 4/mmm (4/m 2/m 2/m) : I41/amd |
| Jacobsite | Mn2+Fe3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Jacobsite-Q | Mn2+(Fe3+,Mn3+)2O4 | Tet. 4/mmm (4/m 2/m 2/m) : I41/amd |
| Maghemite | (Fe3+0.67◻0.33)Fe3+2O4 | Iso. 4 3 2 : P41 3 2 |
| Magnesiochromite | MgCr2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Magnesiocoulsonite | MgV2O4 | Iso. m3m (4/m 3 2/m) |
| Magnesioferrite | MgFe3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Manganochromite | Mn2+Cr2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Nichromite | (Ni,Co,Fe)(Cr,Fe,Al)2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Spinel | MgAl2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Thermaerogenite | CuAl2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Titanomaghemite | (Ti4+0.5◻0.5)Fe3+2O4 | Iso. 4 3 2 : P43 3 2 |
| Trevorite | Ni2+Fe3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| UM1994-06-O:AlCo | CoAl2O4 | |
| Vuorelainenite | Mn2+V3+2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
| Zincochromite | ZnCr2O4 | Iso. m3m (4/m 3 2/m) : Fd3m |
Forms a series with:
Common Associates
Associated Minerals Based on Photo Data:
| 594 photos of Magnetite associated with Calcite | CaCO3 |
| 473 photos of Magnetite associated with Quartz | SiO2 |
| 411 photos of Magnetite associated with Hematite | Fe2O3 |
| 317 photos of Magnetite associated with Chalcopyrite | CuFeS2 |
| 293 photos of Magnetite associated with Pyrite | FeS2 |
| 256 photos of Magnetite associated with Epidote | (CaCa)(AlAlFe3+)O[Si2O7][SiO4](OH) |
| 186 photos of Magnetite associated with Clinochlore | Mg5Al(AlSi3O10)(OH)8 |
| 166 photos of Magnetite associated with Fluorapatite | Ca5(PO4)3F |
| 159 photos of Magnetite associated with Dolomite | CaMg(CO3)2 |
| 151 photos of Magnetite associated with Andradite | Ca3Fe3+2(SiO4)3 |
Related Minerals - Strunz-mindat Grouping
| 4.BB. | Elgoresyite | (Mg5Si2)O9 |
| 4.BB. | Chenmingite | FeCr2O4 |
| 4.BB. | Dellagiustaite | V2+Al2O4 |
| 4.BB. | Thermaerogenite | CuAl2O4 |
| 4.BB. | Chukochenite | LiAl5O8 |
| 4.BB. | Garpenbergite | Mn6◻AsSbO10(OH)2 |
| 4.BB. | Magnéliite | Ti3+2Ti4+2O7 |
| 4.BB. | Jianmuite | ZrTi4+Ti3+5Al3O16 |
| 4.BB.05 | Chromite | Fe2+Cr3+2O4 |
| 4.BB.05 | Cochromite | CoCr2O4 |
| 4.BB.05 | Coulsonite | Fe2+V3+2O4 |
| 4.BB.05 | Cuprospinel | Cu2+Fe3+2O4 |
| 4.BB.05 | Filipstadite | (Fe3+0.5Sb5+0.5)Mn2O4 |
| 4.BB.05 | Franklinite | Zn2+Fe3+2O4 |
| 4.BB.05 | Gahnite | ZnAl2O4 |
| 4.BB.05 | Galaxite | Mn2+Al2O4 |
| 4.BB.05 | Hercynite | Fe2+Al2O4 |
| 4.BB.05 | Jacobsite | Mn2+Fe3+2O4 |
| 4.BB.05 | Manganochromite | Mn2+Cr2O4 |
| 4.BB.05 | Magnesiocoulsonite | MgV2O4 |
| 4.BB.05 | Magnesiochromite | MgCr2O4 |
| 4.BB.05 | Magnesioferrite | MgFe3+2O4 |
| 4.BB.05 | Nichromite | (Ni,Co,Fe)(Cr,Fe,Al)2O4 |
| 4.BB.05 | Qandilite | (Mg,Fe3+)2(Ti,Fe3+,Al)O4 |
| 4.BB.05 | Spinel | MgAl2O4 |
| 4.BB.05 | Trevorite | Ni2+Fe3+2O4 |
| 4.BB.05 | Ulvöspinel | TiFe2+2O4 |
| 4.BB.05 | Vuorelainenite | Mn2+V3+2O4 |
| 4.BB.05 | Zincochromite | ZnCr2O4 |
| 4.BB.05 | Harmunite | CaFe2O4 |
| 4.BB.05 | Wernerkrauseite | Ca(Fe3+,Mn3+)2Mn4+O6 |
| 4.BB.05 | Deltalumite | (Al0.67◻0.33)Al2O4 |
| 4.BB.05 | Guite | Co2+Co3+2O4 |
| 4.BB.10 | Hausmannite | Mn2+Mn3+2O4 |
| 4.BB.10 | Hetaerolite | ZnMn2O4 |
| 4.BB.10 | Manganostibite | (Mn,Fe)7SbAsO12 |
| 4.BB.15 | Maghemite | (Fe3+0.67◻0.33)Fe3+2O4 |
| 4.BB.15 | Titanomaghemite | (Ti4+0.5◻0.5)Fe3+2O4 |
| 4.BB.20 | Tegengrenite | (Mn3+0.5Sb5+0.5)Mg2O4 |
| 4.BB.25 | Xieite | Fe2+Cr2O4 |
| 4.BB.30 | Carmeltazite | ZrAl2Ti4O11 |
| 4.BB.35 | Feiite | Fe2+2(Fe2+Ti4+)O5 |
| 4.BB.40 | Maohokite | MgFe2O4 |
| 4.BB.45 | Tschaunerite | (Fe2+)(Fe2+Ti4+)O4 |
Fluorescence of Magnetite
Not fluorescent in UV.
Other Information
Magnetism:
Ferromagnetic
Thermal Behaviour:
Before the blowpipe, fusible with great difficulty. In an oxidizing flame, loses its magnetic properties.
Notes:
Soluble in HCl.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Industrial Uses:
Ore of iron.
Magnetite in petrology
An essential component of rock names highlighted in red, an accessory component in rock names highlighted in green.
- Ore
- Igneous rock
- Sedimentary rock and sediment
- Metamorphic rock
Internet Links for Magnetite
mindat.org URL:
https://www.mindat.org/min-2538.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Magnetite
Significant localities for Magnetite
Showing 17 significant localities out of 18,570 recorded on mindat.org.
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Australia | |
| Whitehead +3 other references |
Canada | |
| Ontario Ministry of Northern ... +2 other references |
| Olivier Langelier Collection +1 other reference |
France | |
| Pierrot R. et al. (1975) |
Italy | |
| Gruppo Mineralogico Lombardo (10) +1 other reference |
Norway | |
| [var: Vanodiochrome spinel] Mathiesen (1969) |
Portugal | |
| Collected by Rui Nunes in several ... |
UK | |
| [Specimen in the Natural History Museum |
USA | |
| Min Rec 35:5 pp383-404 |
| Williams (circa 1945) +2 other references |
| Schooner (circa 1985) | |
| Dale (1910) |
| Dunn (1995) +1 other reference |
| - (Dana, 1874) +1 other reference |
| Rocks & Min.: 13:213. +1 other reference |
| Miller (1971) |
| Hawley (1934) |
| Hawley (1934) |
Quick NavTopAbout MagnetiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystallographic forms Crystal StructureEpitaxial Relationships X-Ray Powder DiffractionGeological EnvironmentSynonymsOther LanguagesVarietiesRelationshipsCommon AssociatesStrunz-MindatFluorescence Other InformationMagnetite in petrologyInternet Links References Significant localities Locality List






 symbol to view information about a locality.
The
symbol to view information about a locality.
The 





Rubiana, Metropolitan City of Turin, Piedmont, Italy