Matildite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Matildite
Formula:
AgBiS2
Colour:
Iron-black to gray
Lustre:
Metallic
Hardness:
2½
Specific Gravity:
6.9
Crystal System:
Trigonal
Name:
From the type locality, the Matilda Mine, near Morococha, Peru.
Dimorph of:
Matildite Group.
Corresponds to the low-temperature (beta) form of synthetic AgBiS2. The high-temperature (alpha) form is only stable above 195 +- 5°C (Wu, 1989).
Solid solution between matildite and galena is complete above 215 +- 15°C; below this temperature characteristic Widmanstätten structure-like textures are formed through exsolution (Craig, 1967). Also forms a solid solution with bohdanowiczite.
Corresponds to the low-temperature (beta) form of synthetic AgBiS2. The high-temperature (alpha) form is only stable above 195 +- 5°C (Wu, 1989).
Solid solution between matildite and galena is complete above 215 +- 15°C; below this temperature characteristic Widmanstätten structure-like textures are formed through exsolution (Craig, 1967). Also forms a solid solution with bohdanowiczite.
Unique Identifiers
Mindat ID:
2592
Long-form identifier:
mindat:1:1:2592:4
GUID
(UUID V4):
(UUID V4):
9a036ba2-8f35-42d3-a8f0-3b7299155642
IMA Classification of Matildite
Approved, 'Grandfathered' (first described prior to 1959)
Classification of Matildite
2.JA.20
2 : SULFIDES and SULFOSALTS (sulfides, selenides, tellurides; arsenides, antimonides, bismuthides; sulfarsenites, sulfantimonites, sulfbismuthites, etc.)
J : Sulfosalts of PbS archetype
A : Galena derivatives with little or no Pb
2 : SULFIDES and SULFOSALTS (sulfides, selenides, tellurides; arsenides, antimonides, bismuthides; sulfarsenites, sulfantimonites, sulfbismuthites, etc.)
J : Sulfosalts of PbS archetype
A : Galena derivatives with little or no Pb
3.7.1.1
3 : SULFOSALTS
7 : ø = 2
3 : SULFOSALTS
7 : ø = 2
5.2.17
5 : Sulphosalts - Sulpharsenites and Sulphobismuthites (those containing Sn, Ge,or V are in Section 6)
2 : Sulpharsenites etc. of Ag
5 : Sulphosalts - Sulpharsenites and Sulphobismuthites (those containing Sn, Ge,or V are in Section 6)
2 : Sulpharsenites etc. of Ag
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Mtd | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Matildite
Metallic
Transparency:
Opaque
Colour:
Iron-black to gray
Streak:
Light gray
Hardness:
2½ on Mohs scale
Tenacity:
Brittle
Cleavage:
None Observed
Fracture:
Irregular/Uneven
Density:
6.9 g/cm3 (Measured) 6.9 g/cm3 (Calculated)
Optical Data of Matildite
Anisotropism:
Weak
Reflectivity:
| Wavelength | R1 | R2 |
|---|---|---|
| 400nm | 41.4% | 47.2% |
| 420nm | 41.9% | 47.8% |
| 440nm | 42.2% | 48.3% |
| 460nm | 43.1% | 48.8% |
| 480nm | 43.4% | 49.3% |
| 500nm | 43.4% | 49.3% |
| 520nm | 43.0% | 49.0% |
| 540nm | 42.8% | 48.4% |
| 560nm | 42.5% | 47.8% |
| 580nm | 42.2% | 47.3% |
| 600nm | 41.7% | 46.9% |
| 620nm | 41.3% | 46.7% |
| 640nm | 40.8% | 46.7% |
| 660nm | 40.3% | 46.7% |
| 680nm | 39.9% | 46.3% |
| 700nm | 39.3% | 46.1% |
Graph shows reflectance levels at different wavelengths (in nm). Top of box is 100%. Peak reflectance is 49.3%.
R1 shown in black, R2 shown in red
Colour in reflected light:
White
Pleochroism:
Weak
Chemistry of Matildite
Mindat Formula:
AgBiS2
Elements listed:
Crystallography of Matildite
Crystal System:
Trigonal
Class (H-M):
3 2 - Trapezohedral
Space Group:
P31 2 1
Cell Parameters:
a = 4.0675(3) Å, c = 18.9570(13) Å
Ratio:
a:c = 1 : 4.661
Unit Cell V:
271.62 ų (Calculated from Unit Cell)
Morphology:
Rarely as indistinct prismatic crystals, striated [001]. Massive, granular.
Comment:
Space group and cell from Kolitsch et al. (2021). Originally assumed to have space group P-3m1. Note: Harris & Thorpe (1969) suggested a hexagonal cell with a = 8.12, c = 19.02 Å.
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0009218 | Matildite | Geller S, Wernick J H (1959) Ternary semiconducting compounds with sodium chloride-like structure: AgSbSe2, AgSbTe2, AgBiS2, AgBiSe2 Acta Crystallographica 12 46-54 |  | 1959 | synthetic | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 2.827 Å | (100) |
| 3.302 Å | (80) |
| 1.966 Å | (60) |
| 2.029 Å | (50) |
| 6.311 Å | (30) |
| 1.709 Å | (30) |
| 3.453 Å | (20) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| High-𝑇 alteration and/or metamorphism | |
| 33 : Minerals deposited by hydrothermal metal-rich fluids (see also [#12]) |
Type Occurrence of Matildite
Synonyms of Matildite
Other Language Names for Matildite
Japanese:マティルダ鉱
Spanish:Argentobismutita
Matildita
Matildita
Varieties of Matildite
| Cupromatildite | Cu-bearing variety of matildite. A mechanical mixture? |
Relationship of Matildite to other Species
Structurally related to group(s):
Common Associates
Associated Minerals Based on Photo Data:
| 44 photos of Matildite associated with Chalcopyrite | CuFeS2 |
| 35 photos of Matildite associated with Quartz | SiO2 |
| 27 photos of Matildite associated with Galena | PbS |
| 21 photos of Matildite associated with Arsenopyrite | FeAsS |
| 21 photos of Matildite associated with Bismuth | Bi |
| 16 photos of Matildite associated with Pyrite | FeS2 |
| 11 photos of Matildite associated with Pyrrhotite | Fe1-xS |
| 6 photos of Matildite associated with Rhabdophane-(Ce) | Ce(PO4) · 0.6H2O |
| 4 photos of Matildite associated with Bismuthinite | Bi2S3 |
| 4 photos of Matildite associated with Benjaminite | Ag3Bi7S12 |
Related Minerals - Strunz-mindat Grouping
| 2.JA. | Ferdowsiite | Ag8(Sb5As3)S16 |
| 2.JA. | Sangenaroite | Ag8(Sb8-xAsx)SΣ16 |
| 2.JA. | Luboržákite | Mn2AsSbS5 |
| 2.JA.05e | Benjaminite | Ag3Bi7S12 |
| 2.JA.05g | Borodaevite | Ag5(Bi,Pb,Fe)8(Sb,Bi)2S17 |
| 2.JA.05a | Cupropavonite | Cu0.9Ag0.5Pb0.6Bi2.5S5 |
| 2.JA.05i | Livingstonite | HgSb4S6(S2) |
| 2.JA.05d | Makovickyite | Cu1.12Ag0.81Pb0.27Bi5.35S9 |
| 2.JA.05f | Mummeite | Cu0.58Ag3.11Pb1.10Bi6.65S13 |
| 2.JA.05a | Pavonite | AgBi3S5 |
| 2.JA.05b | Grumiplucite | HgBi2S4 |
| 2.JA.05h | Mozgovaite | PbBi4S7 |
| 2.JA.05d | Cupromakovickyite | Cu4AgPb2Bi9S18 |
| 2.JA.05c | Kudriavite | (Cd,Pb)Bi2S4 |
| 2.JA.05a | Cupromakopavonite | Ag3Cu8Pb4Bi19S38 |
| 2.JA.05 | Dantopaite | Ag5Bi13S22 |
| 2.JA.05 | Selenodantopaite | Ag5Bi13Se22 |
| 2.JA.10a | Cuprobismutite | Cu8AgBi13S24 |
| 2.JA.10c | Hodrušite | Cu8Bi12S22 |
| 2.JA.10e | Padĕraite | Cu7[(Cu,Ag)0.33Pb1.33Bi11.33]S22 |
| 2.JA.10d | Pizgrischite | (Cu,Fe)Cu14PbBi17S35 |
| 2.JA.10b | Kupčíkite | Cu3.4Fe0.6Bi5S10 |
| 2.JA.15 | Schapbachite | Ag0.4Pb0.2Bi0.4S |
| 2.JA.15 | Cuboargyrite | AgSbS2 |
| 2.JA.20 | Bohdanowiczite | AgBiSe2 |
| 2.JA.20 | Volynskite | AgBiTe2 |
Other Information
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Matildite
mindat.org URL:
https://www.mindat.org/min-2592.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Matildite
Localities for Matildite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Argentina | |
| Hubert et al. (2009) |
| DE BRODTKORB +1 other reference |
| BRODTKORB | |
| Sureda et al. (2010) |
| DE BRODTKORB +1 other reference |
| Paar et al. (2000) |
| DE BRODTKORB +2 other references |
| February 2009 +3 other references |
Australia | |
| Henley et al. (2001) |
| Henley et al. (2001) | |
| Mumme (1975) |
| Skwarnecki (2004) |
| Bottrill et al. (2008) |
| Bottrill et al. (2008) | |
Austria | |
| Michael Waitzinger et al. (2021) |
| Paar et al. (1998) |
| Feitzinger et al. (1991) |
| Niedermayr et al. (1995) | |
| Paar et al. (1982) +1 other reference |
| Pichler (2009) |
| Niedermayr et al. (1999) |
| Pichler (2009) | |
| PICHLER (2009) | |
| Niedermayr et al. (1995) | |
| Strasser (1989) |
| [Lapis 1993:5 p.13-28] +1 other reference |
| Horner et al. (1997) | |
| Horner et al. (1997) | |
| Leitner (2013) |
| Raith et al. (2015) | |
| Exel (1993) |
| Kolitsch (2015) |
Bolivia | |
| USGS Bulletin # 1975 |
| Cacho et al. (2015) |
| Cacho et al. (2019) | |
| Cacho et al. (2015) | |
| Franco (2018) | |
| Jiménez-Franco et al. (2018) | |
| Jiménez-Franco et al. (2018) | |
| Federico Ahlfeld and Alejandro Schneider-Scherbina (1964) +1 other reference | |
| Dana 7:I:430. |
| USGS Bulletin # 1975 |
| Ahlfeld et al. (1964) | |
| Smith et al. (2001) |
| Lindgren et al. (1928) |
Brazil | |
| Silva (2017) |
Bulgaria | |
| Cook et al. (2002) +1 other reference |
| Munkov (1968) |
| [MinRec 22 p.439] |
| www.bgd.bg (n.d.) +1 other reference |
Canada | |
| Peatfield (n.d.) |
| Hood et al. (1991) |
| Anthony et al. (1990) |
| Examination of Micro-Scale Inter- and ... +1 other reference |
| D.V. Venugopal (1985) |
| Huminicki et al. (2008) |
| Can Min 9:665-662 +1 other reference |
| Badham (1975) | |
| Badham (1975) | |
| D. C. Harris (1973) +1 other reference | |
| Rich et al. (1977) +1 other reference |
| Dennis (1987) |
| Grant (2009) |
| Gemmell (2013) |
| Ames et al. (TGI) +1 other reference |
| Ames et al. (2003) |
| Kjarsgaard et al. (2010, June) | |
| Kjarsgaard et al. (2010, June) |
| Péntek et al. (2013) | |
| Kjarsgaard et al. (2010, June) | |
| 157-158. +1 other reference |
| 159-161. +1 other reference |
| Traill R. J. (1983) |
| the Canadian Mineralogist V 11 Part 1 ... | |
| J. D. Scott (1976) | |
| Ellsworth | |
| Ontario and Quebec. Canadian Geological ... +2 other references | |
| Petruk (1971) |
| Alexander (2021) |
| Barkov et al. (2002) |
Chile | |
| Neues Jahrbruch Fur Mineralogie +1 other reference |
| Sernageomin database 2005 +1 other reference | |
China | |
| Weining Zhou (1994) |
| Yi Li and Youbin Liang (1993) |
| Jianping Zhou et al. (1996) |
| Bin Yu (2005) |
| Wenfeng Zhu et al. (2009) |
| Xilin (1990) +1 other reference |
| Mineral Deposits 4 (1) | |
| Hongyuan Xia et al. (1991) |
| Jinjie Yu et al. (2013) |
| Yunqing Yi et al. (1986) |
| Nan et al. (2023) |
| Siwei He and Minyun Sun (1986) +1 other reference |
| Weining Zhou (1994) |
| Zhicheng Lu et al. (2002) |
| Yan Liu et al. (2011) |
| Danyang Liu (1991) |
| Yan Liu et al. (2011) | |
| Yuhua Xie and Jiayin Zhang (1990) +1 other reference |
| Weining Zhou (1994) +2 other references |
| Xia Ai and Zengyi Chen (1993) |
| Liao Shen (1996) |
| Zhenru Zhang et al. (1987) |
| Zhenru Zhang et al. (1987) | |
| Zhenru Zhang et al. (1987) |
| Yongwen Mei (1994) |
| Xianchang Luo (1983) +1 other reference |
| Deqing Xu (1993) | |
| Weining Zhou (1994) |
| Yue et al. (2024) |
| Aixiang Sun (1996) |
| Leiming Xu et al. (1993) +1 other reference |
| Xiang Chi (1999) |
| Liyan Zhang (1985) |
| Sun et al. (2022) |
| Wei et al. (2022) |
| Yuan Zhang et al. (2008) | |
| Chenglu Li et al. (2011) |
| Yuanfu Xiao et al. (2010) |
| Yan Liu et al. (2010) |
| Zhou et al. (2018) |
| Zhou et al. (2016) +1 other reference |
| Peng et al. (1998) |
| Xinpei Jiang (1994) +1 other reference |
| Runsheng Han et al. (2007) |
| Runsheng Han et al. (2007) | |
Cuba | |
| Torró et al. (2016) |
Czech Republic | |
| Pažout et al. (2017) |
| Pazout et al. (2017) |
| Breiter K. |
| Lapis 2002 (7/8) |
| Ondruš et al. (2003) | |
| |
| Hyrsl et al. (2009) | |
| Pauliš P et al. (2020) |
Finland | |
| Hietanen (2015) |
| Novoselov et al. (2015) |
| Clark +1 other reference |
France | |
| |
| Chauris (2014) |
| R. Pierrot |
| P&T N°23-24 et al. (Haut-Rhin) |
| Wittern +1 other reference |
| This mine worked the same vein as Gabe ... +2 other references | |
| Wittern et al. (Cologne) |
| P. Picot |
| Berbain et al. (2005) |
| Moëlo et al. (1989) |
| R. Pierrot |
Germany | |
| Staude et al. (2010) |
| abstract in Mitt. Österr. Mineral. ... +6 other references |
| Walenta (1992) |
| Walenta (1987) |
| |
| Wittern (2001) +1 other reference |
| Walenta (1992) +1 other reference | |
| J. Gröbner: Neufunde von der Grube Katharina im Wildschapbach (2/2004) | |
| Belendorff (2021) |
| Weiß (1990) |
| |
| Weiß (1990) |
| Dill et al. (2010) | |
| Förster et al. (2005) |
| Wittern (2001) |
| Lapis 30 (7/8) | |
| Witzke et al. (2007) |
Greece | |
| Voudouris et al. (2008) |
| VOUDOURIS (2005) | |
| Voudouris et al. (2010, April) +1 other reference |
| Triantafyllidis (2019) |
| Voudouris et al. (2018, July) |
Greenland | |
| Petersen et al. (1993) |
Hungary | |
| Koch: Minerals of Hungary |
Iran | |
| Karimpour et al. (2005) +1 other reference |
| Mehrabi et al. (2016) |
Ireland | |
| Conference +2 other references |
Italy | |
| Vergani F. (2019) |
| Huttenlocher (1934) +2 other references |
| Krismer et al. (2013) |
| Bianchini +5 other references |
Japan | |
| Hariya +1 other reference |
| Min. Jour. (Japan) |
| Shimizu et al. (1998) |
| Canadian Mineralogist Vol. 25 et al. (1987) |
| Akasaka (2007) |
| Akasaka et al. (2007, August) +1 other reference | |
| Dana 7:I:430. +2 other references |
| Nagashima et al. (2021) |
| Nagashima et al. (2016) | |
Kazakhstan | |
| Kovalev et al. (2018) |
| Глоба В.А. Месторождение Манка - новый геолого-промышленный тип месторождений золота. // Изв. НАН РК et al. (403) |
Malaysia | |
| Hoe et al. (2003) |
Mexico | |
| Mining company data |
| Panczner (1987) +1 other reference | |
| Stone +3 other references |
| Canet et al. (2009) +1 other reference |
Morocco | |
| Ilmen et al. (2016) |
Norway | |
| Oftedal (1942) +1 other reference |
Peru (TL) | |
| Dana 7:I:430. +1 other reference |
| 7th Biennial +1 other reference |
| Bendezu et al. (2009) |
| Harlaux et al. (2023) |
Poland | |
| Muszyński et al. (2023) |
| Mederski et al. (2020) |
| Mochnacka et al. (2015) | |
| Gołębiowska B. et al. (Ag) |
| Mochnacka et al. (2000) +2 other references |
| Kozłowski et al. (2022) |
| Mederski et al. (2020) |
Portugal | |
| Antunes et al. (2010) +1 other reference |
| LNEG - Laboratório Nacional de Energia ... |
| LNEG - Laboratório Nacional de Energia ... |
| Gomes (2000) |
| |
| D. Wimmers (1985) |
| Leal Gomes et al. (2009) |
| Mercedes Fuertes-Fuente et al. (2016) |
| Sousa et al. (1987) |
| Gaspar +2 other references |
Romania | |
| Cook et al. (2002) |
| Cook et al. (2003) +1 other reference | |
| Cook et al. (2002) | |
| Cook +1 other reference |
| Mitt. .Österr. Miner. Ges. 143 (1998) |
| Damian et al. (2008) |
| Plotinskaya et al. (2009) |
Russia | |
| Pekov et al. (2011) |
| Vakh et al. (2016) |
| Damdinova et al. (2021) |
| Rogov et al. (2023) |
| Rogov et al. (2023) | |
| Plotinskaya et al. (2018) |
| [Der Aufschluss 1995:4 p.163-180] |
| Kemkina et al. (2018, July) |
| Gonevchuk et al. (2010) |
| Savva et al. (2019) |
| Savva et al. (2019) | |
| Savva et al. (2016, August) +1 other reference | |
| Goryachev et al. (2014) | |
| Grant et al. (2001) +1 other reference |
| Simanenko (2006) +1 other reference | |
| Gvozdev et al. (2014) |
| Ivin et al. (2021) |
| Kuleshevich et al. (2007) |
| Ivashchenko et al. (2011, September) |
| Kondratieva et al. (2021) |
| Kondratieva et al. (2021) | |
| [[1]]Kondratieva et al. (2023) |
| [[1]]Kondratieva et al. (2023) | |
| American Mineralogist +1 other reference |
| Gamyanin et al. (2015) |
| Vikent'eva et al. (2016, April) |
| Kuzhuget et al. (2023) |
| Kiseleva et al. (2008) |
| Prokof’ev et al. (2007) +1 other reference |
| Prokof'ev et al. (2004) +1 other reference | |
Slovakia | |
| Luptáková et al. (2016) |
| |
| Koděra (1986) |
| Ozdín et al. (2004) | |
| Kodě +1 other reference |
| Jeleň et al. (2012) | |
| Mikuš T. (2019) |
| Pršek J. |
South Korea | |
| Dongbok Shin et al. |
Spain | |
| Revista de minerales (Mars) |
| Jimenez R. et al. (2004) |
| Vindel (1980) |
| www.foro-minerales.com (n.d.) |
| Weibel (1955) |
Sweden | |
| Wilke (1997) |
| Wilke (1997) | |
| Wilke (1997) | |
| Wilke (1997) | |
| Wilke (1997) | |
| J.Mansfeld.: ... | |
| Wilke (1997) | |
| Wilke (1997) | |
| MinMag (1992) |
| Nysten |
| Nysten | |
| |
| Erling Olsson and Aksel Østerløf |
Switzerland | |
| Grünenfelder (1957) +2 other references |
| Stalder et al. (1998) |
Tajikistan | |
| Pavlova et al. (2009) |
| Pavlova et al. (2009) | |
| Badalov et al. (1975) |
| Bortnikov et al. (1985) +1 other reference | |
| Badalov et al. (1975) | |
| Badalov et al. (1975) |
| Fayziev et al. (2019) | |
Tunisia | |
| Slim-Shimi et al. (1990) |
Turkey | |
| A. Willgallis et al. : "Se- and ... |
UK | |
| Rollinson et al. (2017) |
| Day (1999) |
| [MinMag 57:751-754] |
| Pattrick (1984) +1 other reference |
| R. E. Bevins (1988) +1 other reference |
USA | |
| USGS PP 1760A |
| Graeme (1993) |
| Graeme (1993) +1 other reference | |
| Stolburg (1984) |
| Hall (1958) +4 other references |
| Foord et al. (1989) | |
| Eckel et al. (1997) |
| Min Rec 18:3 pp185-187 | |
| Eckel et al. (1997) | |
| Eckel et al. (1997) | |
| Eckel et al. (1997) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Genth (1886) +1 other reference |
| Eckel et al. (1997) |
| Eckel et al. (1997) | |
| Shannon et al. (1985) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Holmes et al. (1983) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) | |
| Eckel et al. (1997) | |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Foord et al. (1989) +1 other reference | |
| Dana 7: I:430. |
| Ream (1995) |
| Ream (2004) | |
| MRDS 10241626 |
| - (2005) |
| Cossaboom (1981) +1 other reference |
| - (2005) |
| Castor et al. (2004) | |
| Cooper et al. (1991) |
| R&M 77:5 p298-305 |
| Bullock (1981) |
| Econ Geol (1986) |
| Bullock (1981) |
Quick NavTopAbout MatilditeUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesVarietiesRelationshipsCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List

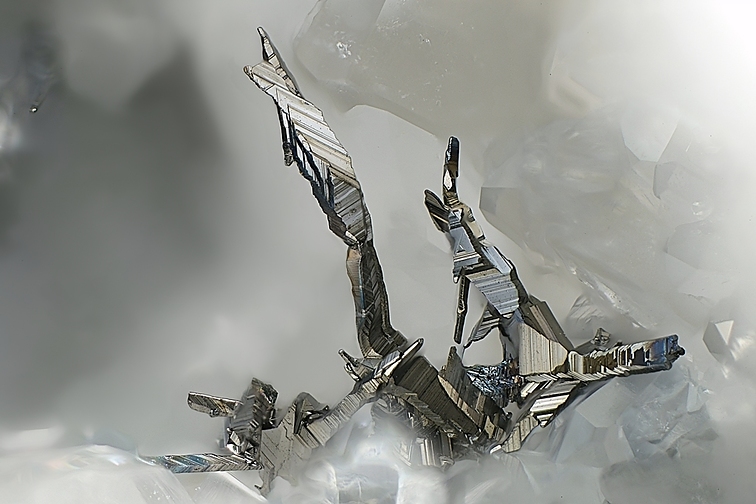




 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Clara Mine, Oberwolfach, Ortenaukreis, Freiburg Region, Baden-Württemberg, Germany