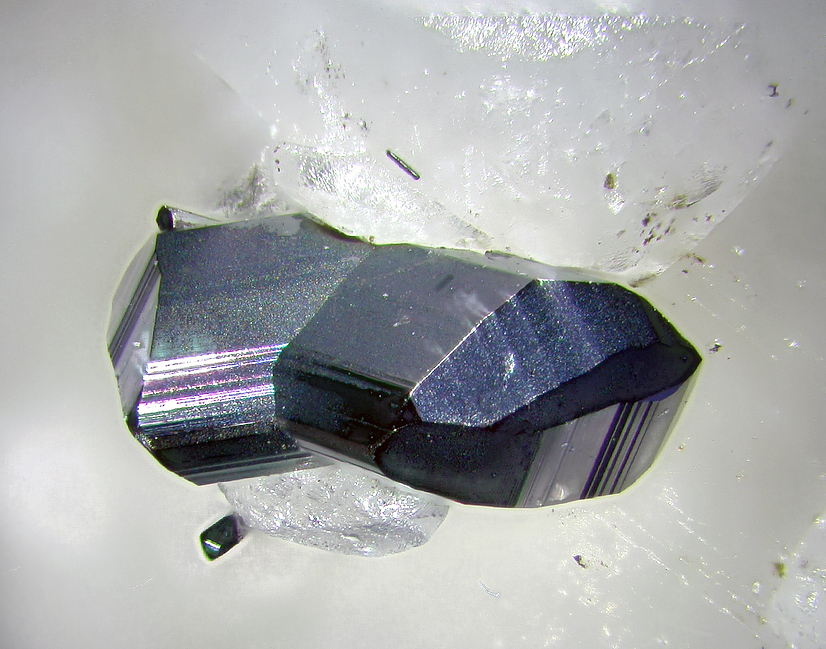
Manganite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Manganite
Formula:
Mn3+O(OH)
Colour:
gray-black, black
Lustre:
Resinous, Sub-Metallic, Dull
Hardness:
4
Specific Gravity:
4.29 - 4.34
Crystal System:
Monoclinic
Name:
Named in 1827 by Wilhelm Karl von Haidinger in allusion to the manganese content.
Polymorph of:
Frequently as well-formed crystals or tight aggregates with a fibrous structure due to the mineral's perfect cleavage. May be compact fine-grained or in botryoidal formation.
Unique Identifiers
Mindat ID:
2519
Long-form identifier:
mindat:1:1:2519:1
GUID
(UUID V4):
(UUID V4):
eff4c35a-f0c3-4f36-92b9-f1c24314884b
IMA Classification of Manganite
Approved, 'Grandfathered' (first described prior to 1959)
Classification of Manganite
4.FD.15
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
F : Hydroxides (without V or U)
D : Hydroxides with OH, without H2O; chains of edge-sharing octahedra
4 : OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates)
F : Hydroxides (without V or U)
D : Hydroxides with OH, without H2O; chains of edge-sharing octahedra
6.1.3.1
6 : HYDROXIDES AND OXIDES CONTAINING HYDROXYL
1 : XO(OH)
6 : HYDROXIDES AND OXIDES CONTAINING HYDROXYL
1 : XO(OH)
7.18.7
7 : Oxides and Hydroxides
18 : Oxides of Mn
7 : Oxides and Hydroxides
18 : Oxides of Mn
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Mnn | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Whitney & Evans (2010) | Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 doi:10.2138/am.2010.3371 | |
| Mnn | Warr (2020) | Warr, L.N. (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Minerals, 55, 261–264 doi:10.1180/clm.2020.30 |
Physical Properties of Manganite
Resinous, Sub-Metallic, Dull
Transparency:
Opaque
Colour:
gray-black, black
Streak:
Reddish brown to black
Hardness:
4 on Mohs scale
Hardness:
VHN100=630 - 743 kg/mm2 - Vickers
Tenacity:
Brittle
Cleavage:
Perfect
{010} perfect; {110} and {001} good.
{010} perfect; {110} and {001} good.
Fracture:
Splintery
Density:
4.29 - 4.34 g/cm3 (Measured) 4.38 g/cm3 (Calculated)
Comment:
Calculated also (ICDD 18-805) = 4.32
Optical Data of Manganite
Type:
Biaxial (+)
RI values:
nα = 2.250(2) nβ = 2.250(2) nγ = 2.530(2)
Birefringence:
0.028
Max Birefringence:
δ = 0.280

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Very High
Anisotropism:
Weak
Bireflectance:
Distinct in grays
Dispersion:
r > v extreme
Optical Extinction:
X = a; Y = b; Z ∧ c = 0°–4°.
Reflectivity:
| Wavelength | R1 | R2 |
|---|---|---|
| 400nm | 18.0% | 25.1% |
| 420nm | 18.0% | 24.8% |
| 440nm | 18.0% | 24.5% |
| 460nm | 17.9% | 24.1% |
| 480nm | 17.8% | 23.7% |
| 500nm | 17.6% | 23.2% |
| 520nm | 17.3% | 22.7% |
| 540nm | 17.0% | 22.2% |
| 560nm | 16.8% | 21.6% |
| 580nm | 16.5% | 21.2% |
| 600nm | 16.3% | 20.8% |
| 620nm | 16.1% | 20.6% |
| 640nm | 16.0% | 20.3% |
| 660nm | 15.8% | 20.1% |
| 680nm | 15.7% | 19.9% |
| 700nm | 15.7% | 19.7% |
Graph shows reflectance levels at different wavelengths (in nm). Top of box is 100%. Peak reflectance is 25.1%.
R1 shown in black, R2 shown in red
Colour in reflected light:
Gray-white with brown tint
Internal Reflections:
Blood-red
Pleochroism:
Weak
Comments:
X = reddish brown; Z = red brown.
Comments:
Weak pleochroism distinguishes manganite from groutite.
Absorption: Z > X = Y.
Absorption: Z > X = Y.
Chemistry of Manganite
Mindat Formula:
Mn3+O(OH)
Elements listed:
Common Impurities:
Fe,Ba,Pb,Cu,Al,Ca
Crystallography of Manganite
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P21/b
Setting:
P21/c
Cell Parameters:
a = 8.94 Å, b = 5.28 Å, c = 5.74 Å
β = 90°
β = 90°
Ratio:
a:b:c = 1.693 : 1 : 1.087
Unit Cell V:
270.95 ų (Calculated from Unit Cell)
Z:
8
Morphology:
Crystals striated and short to long prismatic [001]. Often terminated by {001} alone, by {001} with macrodomes, or by a series of macropyramids; highly modified at times. Prismatic faces deeply striated [001], and terminal {h0l} or {hkl} faces striated parallel to their mutual intersections. Crystals often grouped or markedly composite subparallel [001]. Stalactitic; granular (rare).
Twinning:
1. On {011}, both as contact and penetration twins. Twinning often repeated with composition face either parallel or inclined. 2. Twin plane {100} lamellar, assuming a monoclinic symmetry for the species.
Comment:
Space group B21/d (non-standard setting); pseudo-orthorhombic.
Crystallographic forms of Manganite
Crystal Atlas:
Image Loading
3d models and HTML5 code kindly provided by
www.smorf.nl.
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Toggle
Edge Lines | Miller Indices | Axes
Transparency
Opaque | Translucent | Transparent
View
Along a-axis | Along b-axis | Along c-axis | Start rotation | Stop rotation
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0010565 | Manganite | Buerger M J (1936) The symmetry and crystal structure of manganite, Mn(OH)O Zeitschrift fur Kristallographie 95 163-174 |  | 1936 | Ilfeld, Germany | 0 | 293 |
| 0017927 | Manganite | Garrido J (1935) Structure cristalline de la manganite. _cod_database_code 1011020 Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences 200 69-71 | 1935 | 0 | 293 | ||
| 0013942 | Manganite | Kohler T, Armbruster T, Libowitzky E (1997) Hydrogen bonding and Jahn-Teller distortion in groutite, alpha-MnOOH, and manganite, gamma-MnOOH, and their relations to the manganese dioxides ramsdellite and pyrolusite Journal of Solid State Chemistry 133 486-500 | 1997 | Nchwaning Mine, Kalahari manganese field, Republic of South Africa | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 3.41 Å | (100) |
| 2.65 Å | (50) |
| 2.52 Å | (10) |
| 2.41 Å | (80) |
| 2.36 Å | (2) |
| 2.28 Å | (30) |
| 2.20 Å | (30) |
| 1.781 Å | (20) |
| 1.710 Å | (6) |
| 1.675 Å | (90) |
| 1.637 Å | (2) |
| 1.502 Å | (10) |
| 1.435 Å | (16) |
| 1.323 Å | (6) |
| 1.262 Å | (2) |
| 1.214 Å | (2) |
| 1.182 Å | (2) |
| 1.158 Å | (2) |
| 1.139 Å | (2) |
| 1.118 Å | (2) |
| 1.099 Å | (2) |
| 1.077 Å | (2) |
| 1.015 Å | (2) |
| 0.993 Å | (2) |
Comments:
ICDD 18-805 (synthetic); ICDD 9-99 is superseded
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 3b: Earth’s earliest hydrosphere | >4.45 |
| 14 : Hot springs, geysers, and other subaerial geothermal minerals | |
| Near-surface Processes | |
| 22 : Hydration and low-𝑇 subsurface aqueous alteration (see also #23) | |
| 24 : Authigenic minerals in terrestrial sediments (see also #17) | |
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| 47h : [Near-surface oxidized, dehydrated minerals] | |
| Stage 10a: Neoproterozoic oxygenation/terrestrial biosphere | <0.6 |
| 48 : Soil leaching zone minerals | <0.6 |
Geological Setting:
Low temperature hydrothermal or hot spring manganese deposits. Sedimentary deposits.
Type Occurrence of Manganite
General Appearance of Type Material:
Coarse rectangular prism, often with multiple parallel growth.
Place of Conservation of Type Material:
No designated type specimens.
Geological Setting of Type Material:
Hydrothermal veins
Associated Minerals at Type Locality:
Synonyms of Manganite
Other Language Names for Manganite
Common Associates
Associated Minerals Based on Photo Data:
| 170 photos of Manganite associated with Rhodochrosite | MnCO3 |
| 87 photos of Manganite associated with Baryte | BaSO4 |
| 71 photos of Manganite associated with Calcite | CaCO3 |
| 65 photos of Manganite associated with Quartz | SiO2 |
| 46 photos of Manganite associated with Pyrolusite | Mn4+O2 |
| 32 photos of Manganite associated with Manganese-bearing Calcite | (Ca,Mn)CO3 |
| 28 photos of Manganite associated with Sturmanite | Ca6Fe3+2(SO4)2.5[B(OH)4](OH)12 · 25H2O |
| 27 photos of Manganite associated with Hematite | Fe2O3 |
| 23 photos of Manganite associated with Ettringite | Ca6Al2(SO4)3(OH)12 · 26H2O |
| 23 photos of Manganite associated with Hausmannite | Mn2+Mn3+2O4 |
Related Minerals - Strunz-mindat Grouping
| 4.FD.05 | Spertiniite | Cu(OH)2 |
| 4.FD.10 | Bracewellite | CrO(OH) |
| 4.FD.10 | Diaspore | AlO(OH) |
| 4.FD.10 | Groutite | Mn3+O(OH) |
| 4.FD.10 | Guyanaite | CrO(OH) |
| 4.FD.10 | Montroseite | (V3+,Fe3+)O(OH) |
| 4.FD.10 | Tsumgallite | GaO(OH) |
| 4.FD.20 | Yttrotungstite-(Y) | YW2O6(OH)3 |
| 4.FD.20 | Yttrotungstite-(Ce) | (Ce,Nd,Y)W2O6(OH)3 |
| 4.FD.25 | Frankhawthorneite | Cu2Te6+O4(OH)2 |
| 4.FD.30 | Khinite | Pb2+Cu2+3[Te6+O6](OH)2 |
| 4.FD.30 | Khinite-3T | Pb2+Cu2+3Te6+O6(OH)2 |
| 4.FD.30 | Housleyite | Pb6CuTe6+4O18(OH)2 |
Fluorescence of Manganite
Not fluorescent in ultraviolet.
Other Information
Thermal Behaviour:
In a closed tube, yields water.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Manganite
mindat.org URL:
https://www.mindat.org/min-2519.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Manganite
Reference List:
Kohler, Thomas, Armbruster, Thomas, Libowitzky, Eugen (1997) Hydrogen Bonding and Jahn–Teller Distortion in Groutite,α-MnOOH, and Manganite,γ-MnOOH, and Their Relations to the Manganese Dioxides Ramsdellite and Pyrolusite. Journal of Solid State Chemistry, 133. 486-500 doi:10.1006/jssc.1997.7516
Localities for Manganite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Argentina | |
| Raúl J. Tauber Larry´s collection. |
| DE BRODTKORB +2 other references | |
| Márquez-Zavalía et al. (2016) | |
| C. T. Friz |
| C. T. Friz | |
| Raúl Jorge Tauber Larry +1 other reference | |
| Hugo A. Peña (1970) | |
| Ávila |
Australia | |
| Stevens (1972) |
| Brown et al. (1992) |
| Gilligan et al. (1995) |
| Jones (1955) +1 other reference |
| Jones (1955) +1 other reference | |
| Ostwald (1981) |
| |
| Francis (2010) |
| Record of Mines – Summary Card No:317 |
| Johnston et al. (1978) |
| Noble et al. (1983) |
| Francis et al. (self published) |
| Bottrill et al. (2008) |
| Simpson (1948) |
| Sorrell (n.d.) |
| Jones et al. (2013) | |
| Simpson (1948) |
| Simpson (1948) |
| Putzolu et al. (2018) |
Austria | |
| European Journal of Mineralogy 17 (1) |
| N. Zadorlaky-Stettner: Berg- und Hüttenmännische Monatshefte 107 (10) | |
| H. Meixner: Der Karinthin 9:184-189 (1950) |
| Niedermayr et al. (1995) |
| Meixner (1968) |
| Niedermayr et al. (1995) |
| - (n.d.) +1 other reference |
| Bojar (2006) |
| Bojar (2006) | |
| Taucher (1994) |
| Kolitsch et al. (2012) |
| collected by Peter Arthofer +1 other reference |
| Bernhard Gruber et al. (Berichte) |
Belgium | |
| Malaise (1913) +2 other references |
| Malaise (1913) +3 other references | |
| Malaise (1913) +3 other references |
| |
| Malaise (1913) +2 other references |
| José Dehove collection |
| Malaise (1913) +2 other references |
Bosnia and Herzegovina | |
| Vujanovic (1962) |
Botswana | |
| Yonggang Zhu et al. (2013) |
Brazil | |
| Rezende et al. (2021) |
| |
Bulgaria | |
| Banushev et al. (1994) |
| Kanazirski (1985) |
| Chavdarova et al. (2022) |
| Collection Voigt |
Burkina Faso | |
| Sattran et al. (2002) |
Canada | |
| Peatfield (n.d.) |
| Peatfield (n.d.) |
| Peatfield (n.d.) |
| BC Minfile +2 other references |
| F. Rutley: "Elements of Mineralogy" (1900) |
| F. Rutley: "Elements of Mineralogy" (1900) |
| USGS: Geological Survey Circular 553 +1 other reference |
| Hudgins (1967) |
| Bishop et al. (1974) |
| Harder (1910) |
| USGS: Geological Survey Circular 553 | |
| USGS: Geological Survey Circular 553 | |
| R. A. F. Penrose | |
| |
| Van Dommelen et al. (2006) | |
| USGS: Geological Survey Circular 553 |
| USGS: Geological Survey Circular 553 | |
| Wright (1975) |
| Cook (1999) |
| Ontario Div. Mines et al. (1:31,680) +1 other reference |
| Personal Collection - Bill Morgenstern |
| Bill Morgenstern - Collection | |
| Bill Morgenstern - Collection | |
| Bancroft Mineral Museum +1 other reference |
| 179-181. +2 other references |
| Choquette et al. (2023) |
Chile | |
| Cook (1978) |
| Maurizio Dini - analysed material by ... |
| Samples analysed by Dr. Jochen Schlüter (Hamburg University) |
| Oyarzun et al. (1998) |
| Oyarzun et al. (1998) | |
| C. Ruiz Fuller/F. Peebles (1988) |
| samples analysed by Anthony Kampf +1 other reference |
China | |
| Jiuling Zhang (1982) |
| Nanlai Zheng et al. (1986) +1 other reference |
| Lisheng Luo (1988) | |
| Bin Yu (2005) |
| See also Nowlan +2 other references |
| www.mineralmundi.com (n.d.) |
| Hanquan Ye (1985) |
| Ruixia Hao and Guangyue Guan (1995) |
| Jiuling Zhang (1982) +1 other reference |
| Hunan Metallurgical Geology Research Team 236 (1977) +1 other reference |
| Jiuling Zhang (1982) |
| Jiuling Zhang (1982) |
| Lin Ye and Tiegang Liu (2003) |
| Jiuling Zhang (1982) |
| Ziqiang Wei (1976) |
| Ziqiang Wei (1976) +1 other reference |
| Canhui Luo (1992) |
| Qingchang Meng (1981) |
| Shikun Huang (1990) |
| Baisheng Zhang (1998) |
| Libo Hao et al. (1994) +1 other reference |
| Shikun Huang (1990) |
| Huawen Qi et al. (2007) |
| Jiuling Zhang (1982) |
| Caiwen Cai (1983) |
| Yunhuai Lin (1986) |
| Guilin Institute of Metallurgical Geology et al. (1976) +2 other references |
| Guilin Metallurgical Institute of Geology (1976) +3 other references |
| Xiong Song (1986) |
| Zhongtang Yang et al. (2008) +1 other reference |
| Jiuling Zhang (1982) +2 other references |
| Xingfen Wang (1989) +1 other reference |
| Jiuling Zhang (1982) |
| Guosheng Feng et al. (2006) +1 other reference |
| Pan et al. (2024) |
| Yiyun Wang et al. (2012) |
| Denghong Wang (1996) |
| Jiuling Zhang (1982) |
| Hongjun Liu (1987) +2 other references |
| Hunan Metallurgical Geology Research Team 236 (1977) |
| Du +6 other references |
| Wenxin Yu (1980) +4 other references |
Cuba | |
| www.minsocam.org |
Czech Republic | |
| Lapis 2002 (7/8) |
| Matýsek D. (Outer Western Carpathians) |
| Kruťa T. et al. (1968) |
| Prachař +6 other references |
| Stránský |
DR Congo | |
| Deliens (1996) |
| ... |
Egypt | |
| S. Tosson et al. |
| Manganese Reserves and Resources of the ... |
| Afify et al. (2022) |
Ethiopia | |
| Getaneh et al. (2022) |
| Getaneh et al. (2022) | |
Europe | |
| Petr Fuchs |
Finland | |
| Ilkka Mikkola collection EDS+XRD ... |
| Aarne Laitakari |
France | |
| Belot (1978) |
| De Ascençao Guedes (2003) |
| Wittern et al. (Cologne) |
| Perseil (1975) | |
| Perseil (1975) | |
| Belot (1978) |
| Lacroix (1910) |
| Wittern et al. (Cologne) |
| Wittern et al. (Cologne) |
| Hohh (1994) |
| Hohh (1994) |
| Wittern |
| Belot (1978) +2 other references |
| Belot (1978) |
| C Gineste |
| Patureau et al. (2011) |
| Belot (1978) |
| Belot (1978) | |
| Belot (1978) |
| www.zampano.com (n.d.) |
| TREILLARD Michel collection |
| |
| Inventaire minéralogique de la France ... |
| Le Règne Minéral 47: 5-21 |
| Brugger et al. (1999) |
| De Ascenção Guedes et al. (2002) |
| Belot (1978) +1 other reference |
| Lacroix (1910) |
| Berbain et al. (2005) | |
| Berbain et al. (2005) | |
| Lacroix (1910) | |
| Berbain et al. (2005) |
| LACROIX A: Mineralogie de la France et ... |
| Garric François |
| Bernadi G. (1992) | |
Gabon | |
| Weber et al. (1979) |
Georgia | |
| Varentsov (1996) |
Germany | |
| Weiß (1990) |
| Weiß (1990) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) | |
| Walenta (1992) |
| Walenta (1992) |
| Aufschluss 1987 (8/9) +2 other references |
| Weiß (1990) |
| Magma 1984 (2) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) | |
| Weiß (1990) |
| |
| Weiß (1990) |
| Schnorrer et al. (2002) +1 other reference |
| Weiß (1990) |
| D. Pawlowski: "Mineralfundstellen im Sauerland" (Munich) |
| Rolf Golze +3 other references |
| Wittern (2001) |
| Wittern (2001) +1 other reference |
| Lapis (12) |
| Rolf Golze +3 other references |
| Weiss: "Mineralienfundstellen et al. (Munich) |
| 50. +1 other reference |
| Collection of NHM |
| Lapis (3) |
| Weiß (1990) |
| Wittern (2001) | |
| Der Aufschluss Vol.55 |
| Held et al. (1993) |
| Aufschluß 76/2 & 88/5 |
| Weiß (1990) |
| Brugger et al. (2001) |
| Grünling (1913) |
| Gröbner et al. (2011) |
| Lapis 30 (7/8) |
| Scheven et al. (2022) |
| Rob Lavinsky specimen |
| - (n.d.) |
| |
| www.mineralatlas.eu (n.d.) |
| Wittern (2001) |
| Rüger et al. (1992) | |
Ghana | |
| Mineralium Deposita Volume 34 Issue 3 (1999) |
Greece | |
| |
| |
| Andrew P. Fornadel et al. (2011) |
| Φωτιάδης (2010) |
| Φωτιάδης (2010) |
Honduras | |
| Bersani et al. (2009) |
| Mattioli et al. (2014) | |
Hungary | |
| Sándor et al. (1997) |
| Mineral Species of Hungary |
| Szakáll-Gatter-Szendrei: Mineral ... |
| |
| Varentsov (1996) +1 other reference |
| Koch: Minerals of Hungary |
| Szakáll-Gatter-Szendrei: Mineral ... | |
India | |
| Rasul (1965) |
| Sarkar (2015) |
| Roy et al. (1968) |
| Mineralogical Magazine 1968 36 : ... |
| Yadav (2022) |
Indian Ocean | |
| Wang et al. (2018) |
Indonesia | |
| Atmoko et al. (2018) |
Iran | |
| Econ Geol (1994) |
| Lotfi et al. (2017) |
Ireland | |
| F. Rutley: "Elements of Mineralogy" (1900) |
| Grennan |
Italy | |
| Castello P. (1982) +2 other references |
| Del Caldo et al. (1973) |
| Stoppani et al. (1982) +1 other reference |
| Antofilli |
| Antofilli | |
| Redazionale (2005) |
| 415. +1 other reference | |
| Pipino (1984) |
| Balestra (2016) |
| Palenzona et al. (1988) |
| Antofilli |
| Antofilli | |
| Di Lisi S. | |
| Capitanio A. (2000) |
| Fagnani G. (1946) |
| Vergani et al. (2021) |
| Boni et al. (1977) |
| Boni et al. (1976) |
| Ravagnani (1974) |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) | |
| Piccoli et al. (2007) |
| Bertolani (1976) |
| Conti-Vecchi et al. (1991) |
| Giovanni Scapin Collection | |
| De Michele (1974) |
| Gamboni et al. (2021) | |
| Orlandi (2011) | |
| Stara et al. (1996) |
| Pietracaprina et al. (1987) |
| Alfonso Frías Forcada Collection |
| www.comune.pisa.it (2000) |
| Biagioni et al. (2013) | |
| Orlandi et al. (1997) |
| Bonifazi (2020) |
| Muenchener Micromounter-Lithothek |
| Barsotti et al. (2006) |
| Giuliano bettini collection |
| AA. VV. |
| Mancini (1997) |
| Orlandi P. (2005) |
| R. Meli (1999) |
| C. Betti et al. (1999) |
| Saccardo (2002) +1 other reference |
Jamaica | |
Japan | |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) | |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) | |
| Sadanaga et al. (1974) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Yamada (2005) |
| Nambu et al (1979) |
| Sadanaga et al. (1974) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) +5 other references | |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Toyofumi Yoshimura et al. (1969) |
| Petrov (n.d.) +1 other reference |
| Masutomi Museum specimens (Kyoto) |
| Sadanaga et al. (1974) |
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
| Yamada (2004) |
| Yamada (2004) |
| Petrov (n.d.) |
Kazakhstan | |
| Попов В.А. (2010) |
| Evseev (1995) |
| Evseev (1995) |
Kyrgyzstan | |
| Filippov et al. (2013) |
Luxembourg | |
| MnhnL collection |
Madagascar | |
| Behier (1963) |
Mexico | |
| Panczner (1987) |
| Panczner (1987) |
| Panczner (1987) | |
| Diaz-Unzueta (1987) |
| Rocks & Min.: 56:247. |
| Panczner (1987) | |
| Panczner (1987) |
| Panczner (1987) | |
| Panczner (1987) | |
| Panczner (1987) | |
| Panczner (1987) | |
| Panczner (1987) |
Morocco | |
| Visual identification by French geologist REMY Philippe (pers. comm. Jean Vincent Coureau) |
| Jordi Fabre specimen |
| Thein (1990) |
| FAVREAU G. & DIETRICH J.E. (2006) |
| Joan Rosell (2022) |
Namibia | |
| in the collection of F.J.Emmerich |
| Dunn (1991) |
Nazca Plate | |
| Zawadzki et al. (2022) | |
New Zealand | |
| crownminerals.med.govt.nz (n.d.) |
| Fraser (1910) |
Norway | |
| Neumann (1985) |
| Kjerulf (1878) +1 other reference | |
| Neumann (1985) | |
| Neumann (1985) |
| Neumann (1985) |
| Neumann (1985) |
| Kjerulf (1878) |
Pacific Ocean | |
| Mikhailik et al. (2021) | |
| Volokhina et al. (2020) +3 other references |
Pakistan | |
| Ghauri Mining & Engineering Integrate |
Papua New Guinea | |
| Steger (2015) |
| Carswell (1990) |
| Siedner (1959) |
Poland | |
| Szakáll (2002) |
| Korczyńska-Oszacka B. 1978: Minerały manganu wapieni jurajskich Doliny Chochołowskiej (Tatry, Polska) | |
| Lis et al. (1986) |
| Lis et al. (1986) |
| Ł. Kruszewski PXRD & pXRF data (paper in preparation) |
Portugal | |
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 635Mn) |
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 635Mn) | |
| LNEG - Laboratório Nacional de Energia ... |
| LNEG - Laboratório Nacional de Energia ... | |
| Martins da Pedra collection |
| Martins da Pedra collection | |
Red Sea | |
| Sediment-Hosted Mineral Deposits: ... | |
Romania | |
| Rădulescu Dan |
| D. Radulescu & R. Dimitrescu (1966) |
| Cook N.J. et al. (1997) +1 other reference |
| minerals-of-the-carpathians.eu (2008) +1 other reference |
| minerals-of-the-carpathians.eu (2008) |
| Hîrtopanu (1997) +1 other reference |
Russia | |
| Chukanov et al. (2021) |
| Chukanov et al. (2021) |
| Pavel.M. Kartashov (n.d.) |
| Okrugin et al. (an Example of Mutnovsky Geothermal Area, Southern Kamchatka) +1 other reference |
| USGS Open File Report 2005-1252 p46 +1 other reference |
| Pekin et al. (2010) +1 other reference | |
| Pekin et al. (2010) +1 other reference | |
| Sharkov (2009) |
| Anthony et al. (1997) |
| Svetova et al. (2023) |
| Econ Geol (1995) |
| Kasatkin et al. (2021) |
Rwanda | |
| Bertossa |
Serbia | |
| Pačevski et al. (2016) +1 other reference |
Slovakia | |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) | |
| Koděra (1986) | |
| Grecula (1995) |
| Koděra (1986) |
| Ďuďa (1993) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) | |
| Koděra (1986) |
| Pauliš P. |
| Háber et al. (SGR) | |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) | |
| Koděra (1986) | |
| Grecula (1995) | |
| Pauliš P. | |
| Grecula (1995) | |
| Koděra (1986) | |
| Koděra (1986) | |
| Koděra (1986) |
| Ďuďa R. et al. ( Kotterbach ) |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) |
| Rokovič I. |
| Rokovič I. |
| Mišík |
| Rokovič I. |
| Rokovič I. |
| Rokovič I. | |
| Mišík |
| Rokovič I. et al. (2003) |
| Koděra (1986) |
South Africa | |
| Cors de Brun photo |
| Wilson et al. (1978) |
| Cairncross et al. (1995) +2 other references | |
| Cairncross et al. (1995) | |
| Bruce Cairncross (2000) |
| Cairncross et al. (1995) | |
| Beukes et al. (1995) | |
| Cairncross et al. (1995) | |
South Korea | |
| Suzuki (1943) |
| Suzuki (1943) |
| Suzuki (1943) |
Spain | |
| Calvo (2009) +1 other reference |
| My personal collection. | |
| A. Arribas et al. (2005) |
| Bocamina (4) |
| Dill et al. (2023) |
| Bareche (2005) |
| Mineralogistes de Catalunya |
| Mata Perelló |
Sudan | |
| Yassin et al. (1984) |
| Yassin et al. (1984) | |
Sweden | |
| |
| |
| |
| |
| |
| Dana 6:A3:15. |
| |
| GFF 48 |
| |
| The Swedish Museum of Natural History |
| |
| Langhof et al. (Pt. 2, June) +1 other reference |
| |
| Holtstam et al. (1999) | |
| Gatedal (n.d.) | |
| Palache et al. (1951) | |
| Sandström et al. (2009) |
| Sandström et al. (2009) | |
| |
| |
| |
| |
| Sandtsröm (2002) |
| Sandtsröm (2002) | |
| Sandtsröm (2002) | |
| Sandtsröm (2002) | |
| Sandtsröm (2002) | |
| Sandtsröm (2002) |
| Sven-Olof Niklasson photo & specimen | |
Switzerland | |
| |
| Stalder et al. (1998) |
| Stalder et al. (1998) |
| The Canadian Mineralogist Vol. 44 (2006) +1 other reference |
Tonga | |
| Paropkari et al. (2010) |
Turkey | |
| Öztürk (1997) |
| Öztürk (1997) | |
| Gultekin et al. (2016) |
| TEKER et al. (2006) |
| Öztürk (1997) |
| Öztürk et al. (1995) |
| Oyman et al. (2003) |
| Oksuz (2018) |
| USGS Prof Paper 550C ppC158-C161 | |
UK | |
| Hall (1868) |
| Hall (1868) |
| Rob Bowell (Cardiff University analysis) |
| Golley et al. (1995) |
| Golley et al. (1995) |
| Hall (1868) +2 other references |
| Golley et al. (1995) | |
| P Haas - collected 2009 | |
| Golley et al. (1995) |
| Hall (1868) |
| Greg et al. (1858) +3 other references |
| Day (1999) | |
| Davidson et al. (1951) +1 other reference |
| Davidson et al. (1951) +2 other references |
| Day (1999) |
| Stanley et al. (1991) | |
| |
| Day (1999) | |
| BMS Collection |
| Day (1999) | |
| Collins (1871) |
| Render (n.d.) |
| Royal Cornwall Polytechnic Society (1870) |
| Wolfe et al. (2008) +1 other reference |
| Turner (2006) +1 other reference |
| Day (1999) |
| Burr (1996) |
| Hall (1868) | |
| F. Rutley: "Elements of Mineralogy" (1900) |
| Render (n.d.) | |
| Render (n.d.) |
| Day (1999) |
| Day (1999) |
| |
| Render (n.d.) |
Ukraine | |
| Alexander I. Tischenko (1996) |
| Dvoichenko (1914) +1 other reference |
| Jiuling Zhang (1982) +1 other reference |
| Varentsov (1996) |
USA | |
| Rocks & Min 70:5 pp 320-333 |
| - (1917) | |
| Cook et al. (1982) | |
| - (1917) | |
| - (1917) | |
| Cook et al. (1982) |
| Cook et al. (1982) | |
| Rocks & Min 70:5 pp 320-333 | |
| - (2008) |
| - (2008) |
| - (2008) |
| Mayo (1956) +1 other reference |
| Anthony et al. (1995) |
| Butler et al. (1938b) +5 other references | |
| Rob Bowell | |
| Butler et al. (1938b) +3 other references | |
| Rob Bowell | |
| Christopher O'Neill collection |
| Kiersch (1949) +4 other references |
| Galbraith (1959) +2 other references | |
| Peterson (1962) |
| Jones et al. (1920) +1 other reference |
| Jones et al. (1920) +1 other reference | |
| Peterson (1962) |
| Jones et al. (1920) |
| Anthony et al. (1995) |
| Potter et al. (1946) +1 other reference | |
| Farnham & Stewart (1958) +2 other references |
| Jones et al. (1920) +3 other references | |
| Jones et al. (1920) +1 other reference |
| Galbraith (1959) |
| Farnham & Stewart (1958) +2 other references |
| Havens et al. (1947) +1 other reference |
| Farnham & Stewart (1958) +2 other references |
| Farnham & Stewart (1958) +2 other references | |
| Farnham & Stewart (1958) +1 other reference | |
| Farnham & Stewart (1958) +2 other references |
| Farnham & Stewart (1958) +2 other references | |
| Galbraith (1959) +1 other reference |
| Jones et al. (1920) +1 other reference | |
| Sandell (1948) +1 other reference | |
| MRDS file #10048185. | |
| Jones et al. (1920) +2 other references | |
| Jones et al. (1920) | |
| Jones et al. (1920) +1 other reference | |
| Jones et al. (1920) +1 other reference | |
| MRDS file #10027086. |
| MRDS file #10102433. | |
| Jones et al. (1920) +1 other reference | |
| Jones et al. (1920) +1 other reference | |
| MRDS file #10102467. | |
| MRDS file #10027581. | |
| Sandell (1948) +2 other references |
| Jones et al. (1920) +1 other reference | |
| Wilson (1930) | |
| Jones et al. (1920) |
| Jones et al. (1920) |
| Jones et al. (1920) |
| MRDS file #10027549. |
| Jahns (1952) +1 other reference | |
| Wilson et al. (1930) |
| MRDS file #10027684. +1 other reference | |
| Head (1941) +1 other reference |
| MRDS file #10027075. +1 other reference | |
| MRDS file #10027608. | |
| MRDS file #10102537. +1 other reference | |
| MDS file #10027610. +1 other reference | |
| MRDS file #10027663. +1 other reference | |
| MRDS file #10027666. +1 other reference | |
| MRDS file #10027643. | |
| MRDS file #10102532. |
| MRDS file #10102430. | |
| MRDS file #10027685. | |
| MRDS file #10027671. +1 other reference | |
| MRDS file #10027641. |
| MRDS file #10027667. | |
| MRDS file #10027681. |
| Jones et al. (1920) | |
| Jones et al. (1920) | |
| MRDS file #10027698. +1 other reference |
| Preliminary reconnaissance for uranium in Mohave County et al. (Jun 1970) +1 other reference | |
| |
| Farnham (1961) +1 other reference | |
| Dean et al. (1952) +1 other reference |
| Jones et al. (1920) +1 other reference |
| Jones et al. (1920) |
| Anthony et al. (1995) |
| Collected by C. Lemanski |
| Jones et al. (1920) +1 other reference |
| Barnes et al. (1983) | |
| MRDS database Dep. ID file #10103551 |
| Havens et al. (1954) +2 other references |
| Olson (1966) +1 other reference |
| Jones et al. (1920) +1 other reference |
| Long et al. (1948) +1 other reference |
| Anthony et al. (1995) | |
| Jahns (1952) +1 other reference | |
| Galbraith (1959) +1 other reference |
| Farnham & Stewart (1953) +2 other references |
| Anthony et al. (1995) |
| Anthony et al. (1995) |
| Howard (1987) |
| - (2005) |
| - (2005) |
| Smith (1988) |
| Rocks & min.:63:118 +1 other reference |
| Rocks & Min.:63:118. | |
| Rocks & min.:63:118 +1 other reference | |
| Rocks & min.:63:118 +1 other reference | |
| Rocks & min.:63:118 +1 other reference | |
| Rocks & Min.: 63:118. | |
| Rocks & min.:63:118 +1 other reference |
| Rocks & Min.: 63:118. |
| Rocks & Min.: 63:118. | |
| Rocks & Min.: 63:118. | |
| Rocks & Min.: 63: 118 | |
| Rocks & Min.: 63:118. +1 other reference | |
| Rocks & Min.:63:120. |
| Rocks & Min.:63:120 +1 other reference | |
| Rocks & Min.:63:120 +1 other reference | |
| Rocks & Min.:63:120 +1 other reference | |
| Rocks & Min.:63:120 +1 other reference | |
| Rocks & Min.:63:120 +1 other reference | |
| Rocks & Min.: 63: 120. | |
| Rocks & Min.: 63: 120. | |
| Rocks & Min.: 63:120. | |
| Rocks & Min.: 63:120. | |
| Rocks & Min.: 63:120. | |
| Rocks & Min.: 63:120. | |
| Rocks & Min.: 63:120. | |
| Rocks & Min.: 63:120. | |
| Miser et al. (1920) | |
| - (2005) |
| Maxson (1933) +1 other reference |
| Tucker (1926) +2 other references |
| Trask et al. (1943) +1 other reference |
| Pelletier | |
| - (2005) |
| - (2005) |
| - (2005) |
| Bradley et al. (1918) +1 other reference |
| Bradley et al. (1918) +1 other reference | |
| Woodford et al. (1941) |
| - (2005) |
| Van Nostrand Reinholt Press: 166. +2 other references |
| Van Nostrand Reinholt Press: 166. +2 other references | |
| Saul et al. (1970) +1 other reference | |
| Van Nostrand Reinholt Press: 166. +2 other references | |
| Jones et al. (1919) +1 other reference | |
| Van Nostrand Reinholt Press: 166 +3 other references |
| - (2005) |
| - (2005) |
| - (2005) |
| - (2005) |
| Jahns et al. (1951) |
| USGS Bull 427 |
| Murdoch et al. (1966) |
| - (2005) |
| - (2005) |
| - (2005) | |
| - (2005) | |
| - (2005) | |
| - (2005) | |
| - (2005) |
| - (2005) | |
| - (2005) | |
| - (2005) | |
| Eckel et al. (1997) |
| - (2005) |
| - (2005) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Pearl +1 other reference |
| Eckel et al. (1997) |
| - (2005) |
| Eckel et al. (1997) |
| Ralph Lieser of Pappy’s Beryl Shop +2 other references |
| Rocks & Minerals (1970) +1 other reference | |
| - (1917) |
| - (2005) |
| - (2005) |
| Shkurenko +3 other references |
| King et al. (1994) +1 other reference |
| King et al. (1994) |
| Rocks & Min.: 58: 108. |
| Demark (2000) | |
| Heinrich et al. (2004) | |
| ... | |
| Rocks & Min.: 23:717 +1 other reference |
| Heinrich et al. (2004) |
| Heinrich et al. (2004) | |
| Heinrich et al. (2004) |
| ... | |
| Rocks & Min.: 13:137. |
| Heinrich et al. (2004) |
| ... | |
| Heinrich et al. (2004) |
| Heinrich et al. (2004) | |
| Morris Jr. (1983) |
| - (2005) | |
| Morris Jr. (1983) |
| Heinrich et al. (2004) |
| Moore et al. (1979) |
| Rosemeyer (2022) |
| ... | |
| Heinrich et al. (2004) |
| Morris Jr. (1983) |
| Ronald Bently collection specimen-[Rock ... | |
| D. Maietta - Collection. "Mineralogy of Michigan" et al. (more well-known mines in vicinity) | |
| Morris Jr. (1983) | |
| Heinrich et al. (2004) | |
| Heinrich et al. (2004) | |
| Heinrich et al. (2004) | |
| Morris Jr. (1983) |
| Institute on Lake Superior Geology ... |
| Potter et al. (1979) |
| R&M 48:6 p 420 | |
| Econ Geology (1993) | |
| Gobla (2012) |
| - (2005) | |
| Geology and Ore Deposits of the ... +1 other reference | |
| - (2005) | |
| - (2005) |
| Multiple specimens field collected by ... |
| Analyzed using EDS and XRD single ... +1 other reference | |
| - (2005) | |
| Longwell et al. (1965) |
| Castor et al. (2004) | |
| - (2005) |
| - (2005) |
| Castor et al. (2004) |
| NBMG Bull 99B Geology and Mineral ... |
| Castor et al. (2004) |
| - (2005) |
| - (2005) | |
| USGS Prof Paper 353 +2 other references |
| Seaman (1976) |
| Palache (1935) +1 other reference |
| Peters et al. (1983) | |
| Northrop et al. (1996) |
| [Rocks & Minerals Vol 68 March/April ... |
| Entwistle (1944) |
| NMGS 21st Field Conference | |
| Rocks & Min.: 7:124. |
| - (2005) |
| Griswold (1961) +1 other reference | |
| Griswold (1961) | |
| Griswold (1961) | |
| Henry L. Jicha (1954) +1 other reference |
| USGS Bull 945C +1 other reference | |
| Northrop et al. (1996) +1 other reference | |
| Rocks & Min.: 23:938. |
| Patrick Haynes collection |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| - (2005) |
| Rocks & Min. vol. 72 (1997) |
| Rocks & Min. vol.72 (1997) |
| - (2005) |
| - (2005) |
| - (2005) |
| - (2005) | |
| - (2005) | |
| - (2005) | |
| - (2005) |
| - (2005) |
| Oregon Metal Mines Handbook |
| Oregon Metal Mines Handbook |
| Oregon Metal Mines Handbook | |
| Oregon Metal Mines Handbook |
| Oregon Metal Mines Handbook |
| Oregon Metal Mines Handbook | |
| Miller (1971) |
| Michael W. Kieron collection |
| Roberts et al. (1965) |
| Roberts et al. (1965) | |
| Roberts et al. (1965) |
| Roberts et al. (1965) |
| Roberts et al. (1965) |
| Roberts et al. (1965) |
| Smith et al. (2000) |
| Smith et al. (2000) | |
| Roberts et al. (1965) | |
| Roberts et al. (1965) |
| Roberts et al. (1965) |
| Stose et al. (1923) |
| Stose et al. (1923) |
| Paris (2011) |
| Stose et al. (1923) |
| Stose et al. (1923) |
| Stose et al. (1923) | |
| Stose et al. (1923) |
| Stose et al. (1923) |
| USGS Bulletin 737 +1 other reference | |
| USGS Bulletin 737 +1 other reference | |
| Stose et al. (1923) | |
| Paris (2011) |
| Stose et al. (1923) |
| Stose et al. (1923) | |
| Stose et al. (1923) |
| Stose et al. (1923) |
| Stose et al. (1923) | |
| Rylan Lopez Collection |
| BEG Min Res Circ 73 |
| Bullock (1981) |
| - (2005) |
| Bullock (1981) |
| Bullock (1981) |
| Bullock (1981) |
| Bullock (1981) | |
| - (2005) | |
| Bullock (1981) |
| Bullock (1981) |
| Bullock (1981) | |
| Bullock (1981) | |
| Bullock (1981) | |
| Bullock (1981) | |
| Bullock (1981) |
| Bullock (1981) |
| - (2005) |
| Bullock (1981) | |
| Rocks & Min. Vol. 71 (1996) |
| Dietrich (1990) |
| Rocks & Min.:60:165. |
| - (2005) |
| - (2005) |
| Rocks&Min. 47:596 (1972) |
| Dietrich (1990) |
| Dietrich (1990) |
| Dietrich (1990) |
| - (2005) | |
| Dietrich (1990) | |
| The Northern Virginia Mineral Club (2009) |
| Dietrich (1990) |
| - (2005) |
| - (2005) |
| Dietrich (1990) |
| Dietrich (1990) | |
| Rocks&Min. 47:594 (1972) |
| - (2005) |
| - (2005) |
| - (2005) | |
| Rocks & Min.: 60:168. |
| Dietrich (1990) |
| Dietrich (1990) |
| - (2005) |
| Dietrich (1990) |
| Dietrich (1990) | |
| Dietrich (1990) |
| - (2005) |
| Derkey et al. (1990, November) +1 other reference |
| Nicolay & Stone (1967) |
| Dickey (1938) +2 other references |
| Cordua +1 other reference |
| Page et al. (1956) +2 other references |
Uzbekistan | |
| Belogub et al. (2007) |
Quick NavTopAbout ManganiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystallographic forms Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesCommon AssociatesStrunz-MindatFluorescence Other InformationInternet Links References Localities Locality List






 symbol to view information about a locality.
The
symbol to view information about a locality.
The 




N'Chwaning II Mine, N'Chwaning Mines, Joe Morolong Local Municipality, John Taolo Gaetsewe District Municipality, Northern Cape, South Africa