Brochantite
A valid IMA mineral species - grandfathered
This page kindly sponsored by Douglas Merson
About Brochantite
Formula:
Cu4(SO4)(OH)6
Colour:
Green, emerald green, green-black, light green; bluish green in transmitted light.
Lustre:
Vitreous, Pearly
Hardness:
3½ - 4
Specific Gravity:
3.97
Crystal System:
Monoclinic
Name:
Named in 1824 by Serve-Dieu Abailard "Armand" Lévy in honor of the French geologist and mineralogist, André-Jean-François-Marie Brochant de Villiers [August 6, 1772, Mantes-la-Ville, Yvelines, France - May 16, 1840, Paris, France].
A common secondary copper hydroxy sulphate. Two polytypes, both monoclinic, are known (see below).
May be observed as pseudomorphs after malachite, azurite and langite, and altered to chrysocolla. Acicular varieties may be confused with acicular dioptase.
See also the closely related, less common antlerite.
May be observed as pseudomorphs after malachite, azurite and langite, and altered to chrysocolla. Acicular varieties may be confused with acicular dioptase.
See also the closely related, less common antlerite.
Unique Identifiers
Mindat ID:
779
Long-form identifier:
mindat:1:1:779:9
GUID
(UUID V4):
(UUID V4):
e3dcf8a4-b021-443b-a341-f587c3105cc3
IMA Classification of Brochantite
Approved, 'Grandfathered' (first described prior to 1959)
Classification of Brochantite
7.BB.25
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
B : Sulfates (selenates, etc.) with additional anions, without H2O
B : With medium-sized cations
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
B : Sulfates (selenates, etc.) with additional anions, without H2O
B : With medium-sized cations
Dana 7th ed.:
30.1.3.1
30.1.3.1
30 : ANHYDROUS SULFATES CONTAINING HYDROXYL OR HALOGEN
1 : (AB)m(XO4)pZq, where m:p>2:1
30 : ANHYDROUS SULFATES CONTAINING HYDROXYL OR HALOGEN
1 : (AB)m(XO4)pZq, where m:p>2:1
25.2.7
25 : Sulphates
2 : Sulphates of Cu and Ag
25 : Sulphates
2 : Sulphates of Cu and Ag
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
Please only use the official IMA–CNMNC symbol. Older variants are listed for historical use only.
| Symbol | Source | Reference |
|---|---|---|
| Bct | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
| Bro | The Canadian Mineralogist (2019) | The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). download |
Physical Properties of Brochantite
Vitreous, Pearly
Transparency:
Transparent, Translucent
Comment:
Pearly on cleavages.
Colour:
Green, emerald green, green-black, light green; bluish green in transmitted light.
Streak:
Pale green
Hardness:
3½ - 4 on Mohs scale
Cleavage:
Perfect
On {100} perfect.
On {100} perfect.
Fracture:
Irregular/Uneven, Conchoidal
Density:
3.97 g/cm3 (Measured) 4.09 g/cm3 (Calculated)
Optical Data of Brochantite
Type:
Biaxial (-)
RI values:
nα = 1.728 nβ = 1.771 nγ = 1.800
2V:
Measured: 72° , Calculated: 76°
Max Birefringence:
δ = 0.072

Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Very High
Dispersion:
medium r < v
Pleochroism:
Weak
Comments:
Slight in shades of bluish green.
Chemistry of Brochantite
Mindat Formula:
Cu4(SO4)(OH)6
Elements listed:
Crystallography of Brochantite
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P21/b
Setting:
P21/a
Cell Parameters:
a = 13.08 Å, b = 9.85 Å, c = 6.02 Å
β = 103.35°
β = 103.35°
Ratio:
a:b:c = 1.328 : 1 : 0.611
Unit Cell V:
754.65 ų (Calculated from Unit Cell)
Z:
4
Morphology:
Crystals stout prismatic to acicular [001], elongated [010] at times, or, [100] more rarely; also tabular {001}. Loosely coherent aggregates of acicular crystals; groups and drusy crusts; massive, granular.
Twinning:
On {100} with composition surface {100}, common. The twinned crystals are often symmetrical and pseudo-orthorhombic in appearance.
Comment:
OD structure. The MDO1 polytype (P21/a; cell given above) corresponds to "normal" brochantite. The MDO2 polytype has space group P21/n and a = 12.776, b = 9.869, c = 6.026 A, beta = 90.15 (Merlino et al., 2003).
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0006971 | Brochantite | Merlino S, Perchiazzi N, Franco D (2003) Brochantite, Cu4SO4(OH)6: OD character, polytypism and crystal structures European Journal of Mineralogy 15 267-275 | 2003 | Val Fucinaia, Tuscany, Italy | 0 | 293 | |
| 0006972 | Brochantite | Merlino S, Perchiazzi N, Franco D (2003) Brochantite, Cu4SO4(OH)6: OD character, polytypism and crystal structures European Journal of Mineralogy 15 267-275 | 2003 | Capo Calamita, Elba island, Tuscany, Italy | 0 | 293 | |
| 0018378 | Brochantite | Mills S J, Kampf A R, Pasero M, Merlino S (2010) Discreditation of ''orthobrochantite'' (IMA 78-64) as the MDO1 polytype of brochantite European Journal of Mineralogy 22 453-457 | 2010 | Douglas Hill mine, Yerington, Nevada, USA | 0 | 293 | |
| 0010313 | Brochantite | Helliwell M, Smith J V (1997) Brochantite Acta Crystallographica C53 1369-1371 |  | 1997 | Socorro, New Mexico, USA | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Image Loading
Radiation - Copper Kα
Data courtesy of RRUFF project at University of Arizona, used with permission.
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 6.38 Å | (40) |
| 5.36 Å | (40) |
| 3.90 Å | (85) |
| 3.19 Å | (40) |
| 2.923 Å | (20) |
| 2.678 Å | (50) |
| 2.521 Å | (100) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| 47b : [Sulfates and sulfites] | |
| Stage 10a: Neoproterozoic oxygenation/terrestrial biosphere | <0.6 |
| 50 : Coal and/or oil shale minerals | <0.36 |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 54 : Coal and other mine fire minerals (see also #51 and #56) |
Geological Setting:
In arid climates or in rapidly oxidizing copper sulfide deposits under low acid conditions.
Type Occurrence of Brochantite
Synonyms of Brochantite
Other Language Names for Brochantite
Catalan:Brochantita
Czech:Brochantit
Dutch:Brochantiet
Italian:Brochantite
Polish:Brochantyt
Portuguese:Brochantite
Russian:Брошантит
Slovak:Brochantit
Spanish:Brochantita
Swedish:Krisuvigite
Ukrainian:Брошантит
Common Associates
Associated Minerals Based on Photo Data:
| 528 photos of Brochantite associated with Linarite | PbCu(SO4)(OH)2 |
| 369 photos of Brochantite associated with Malachite | Cu2(CO3)(OH)2 |
| 285 photos of Brochantite associated with Quartz | SiO2 |
| 250 photos of Brochantite associated with Cyanotrichite | Cu4Al2(SO4)(OH)12 · 2H2O |
| 149 photos of Brochantite associated with Azurite | Cu3(CO3)2(OH)2 |
| 138 photos of Brochantite associated with Langite | Cu4(SO4)(OH)6 · 2H2O |
| 125 photos of Brochantite associated with Cerussite | PbCO3 |
| 121 photos of Brochantite associated with Spangolite | Cu6Al(SO4)(OH)12Cl · 3H2O |
| 112 photos of Brochantite associated with Chrysocolla | Cu2-xAlx(H2-xSi2O5)(OH)4 · nH2O, x < 1 |
| 111 photos of Brochantite associated with Cuprite | Cu2O |
Related Minerals - Strunz-mindat Grouping
| 7.BB. | Brumadoite | Cu3(Te6+O4)(OH)4 · 5H2O |
| 7.BB. | Iskandarovite | Sb6O7(SO4)2 |
| 7.BB. | Novikovite | (NH4)4Mo6+2Mo5+2O8(SO4)5 |
| 7.BB.10 | Hauckite | Fe3+3(Mg,Mn2+)24Zn18(SO4)4(CO3)2(OH)81 |
| 7.BB.15 | Antlerite | Cu3(SO4)(OH)4 |
| 7.BB.20 | Dolerophanite | Cu2(SO4)O |
| 7.BB.25 | Ramaccioniite | Cu4[SeO4](OH)6 |
| 7.BB.30 | Vergasovaite | Cu3(SO4)(MoO4,SO4)O |
| 7.BB.30 | Cupromolybdite | Cu3O(MoO4)2 |
| 7.BB.35 | Klebelsbergite | Sb4O4(SO4)(OH)2 |
| 7.BB.35 | Tavagnascoite | Bi4O4(SO4)(OH)2 |
| 7.BB.40 | Schuetteite | Hg2+3(SO4)O2 |
| 7.BB.45 | Paraotwayite | Ni(OH)2-x(SO4,CO3)0.5x |
| 7.BB.50 | Xocomecatlite | Cu3(TeO4)(OH)4 |
| 7.BB.55 | Pauflerite | (V4+O)SO4 |
| 7.BB.60 | Grandviewite | Cu3Al2(SO4)(OH)10 · H2O |
| 7.BB.65 | Timroseite | Pb2Cu5(TeO6)2(OH)2 |
| 7.BB.70 | Glikinite | Zn3O(SO4)2 |
| 7.BB.80 | Mojaveite | Cu6[Te6+O4(OH)2](OH)7Cl |
| 7.BB.85 | Paratimroseite | Pb2Cu4(TeO6)2(H2O)2 |
Other Information
Notes:
Soluble in acids.
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Brochantite
mindat.org URL:
https://www.mindat.org/min-779.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Brochantite
Reference List:
Maskelyne, N. S. (1865) On new Cornish minerals of the brochantite group. The London, Edinburgh, And Dublin Philosophical Magazine And Journal Of Science, S. 4 Vol. 29 (198) 473-476 (as waringtonite)
Mrose, Mary E., Reichen, Laura E. (1965) Evidence for the identity of kamarezite with brochantite. Cu4(SO4)(OH)6. American Mineralogist, 50 (9) 1450-1457
Helliwell, M., Smith, J. V. (1997) Brochantite. Acta Crystallographica Section C Crystal Structure Communications, 53 (10) 1369-1371 doi:10.1107/s0108270197006318
Merlino, Stefano, Perchiazzi, Natale, Franco, David (2003) Brochantite, Cu4SO4(OH)6: OD character, polytypism and crystal structures. European Journal of Mineralogy, 15 (2) 267-275 doi:10.1127/0935-1221/2003/0015-0267
Frost, Ray L., Williams, Peter A., Martens, Wayde, Leverett, Peter, Kloprogge, J. Theo (2004) Raman spectroscopy of basic copper(II) and some complex copper(II) sulfate minerals: Implications for hydrogen bonding. American Mineralogist, 89 (7) 1130-1137 doi:10.2138/am-2004-0726
Lane, M. D. (2007) Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. American Mineralogist, 92 (1) 1-18 doi:10.2138/am.2007.2170
Yoder, C. H., Agee, T. M., Ginion, K. E., Hofmann, A. E., Ewanichak, J. E., Schaeffer, C. D., Carroll, M. J., Schaeffer, R. W., McCaffrey, P. F. (2007) The relative stabilities of the copper hydroxyl sulphates. Mineralogical Magazine, 71 (5) 571-577 doi:10.1180/minmag.2007.071.5.571
Crichton, W. A., Muller, H. (2008) Brochantite-2M2 from Pierre Plate Mine, Vizille. Powder Diffraction, 23 (3) 246-250 doi:10.1154/1.2964219
Mills, Stuart J., Kampf, Anthony R., Pasero, Marco, Merlino, Stefano (2010) Discreditation of "orthobrochantite" (IMA 7864) as the MDO1 polytype of brochantite. European Journal of Mineralogy, 22 (3) 453-457 doi:10.1127/0935-1221/2010/0022-2029
Girgsdies, Frank, Behrens, Malte (2012) On the structural relations of malachite. II. The brochantite MDO polytypes. Acta Crystallographica Section B Structural Science, 68 (6) 571-577 doi:10.1107/s0108768112039274
Localities for Brochantite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Afghanistan | |
| Orris et al. (2002) |
| Orris et al. (2002) |
Algeria | |
| Palache et al. (1951) |
| geo.web.ru (n.d.) | |
Antarctica | |
| Vennum +1 other reference |
Argentina | |
| Milka K. de Brodtkorb (2002) |
| - (n.d.) | |
| Colombo et al. (2011) |
| Mr. Nelson Valenzuela. |
| 4 Congreso Nacional y 1 Congreso ... +1 other reference |
| de Brodtkorb +2 other references |
| Garrido +4 other references |
| PONS |
| Brodtkorb (2006) |
| Dill et al. (2013) |
| Brodtkorb (2002) |
Atlantic Ocean | |
| Gablina et al. (2006) |
Australia | |
| |
| McQueen +2 other references | |
| Michael Hirst Collection |
| B. G. Lottermoser: P. M. Ashley (1997) |
| Henley et al. (2001) |
| Rankin et al. (2002) |
| Graham et al. (2005) |
| A.L. McLean et al. (2004) |
| Australia and New Zealand Micromineral ... |
| Chapman et al. (2005) +1 other reference |
| B. G. Lottermoser: P. M. Ashley (1997) +1 other reference |
| Birch et al. (1997) |
| Birch et al. (1997) |
| Worner et al. (et al) +1 other reference |
| Vera Munro-Smith (2006) | |
| Uwe Kolitsch collection/observation |
| Ryan (1961) |
| Ryan (1961) | |
| Collection of RJ Martin inferred from ... |
| Tate Museum Collection |
| Vera Munro-Smith (2006) |
| Day et al. (1996) |
| Dieter Mylius collection |
| Munro-Smith et al. (2015) |
| Day (1998) | |
| Sorrell (n.d.) | |
| Uwe Kolitsch collection | |
| Vera Munro-Smith (2006) |
| |
| Haupt |
| Harris et al. (2003) | |
| Pring et al. (2000) |
| Coats & Blissett (1971) |
| Personal Collection of Mark Willoughby |
| Beyer et al. (1996) |
| Pring et al. (1994) |
| Kolitsch et al. (1999) |
| Vera Munro-Smith (2006) |
| Noble R.J. +1 other reference |
| Sorrell (n.d.) |
| Bottrill et al. (2008) +1 other reference |
| Bottrill et al. (2008) |
| Bottrill & Taheri |
| Bottrill (2018) |
| Bottrill et al. (2020) |
| Bottrill et al. (2008) | |
| Bottrill |
| Bottrill et al. (2008) |
| Bottrill et al. (2008) |
| Bottrill et al. (2008) |
| M. Latham Collection 2011 |
| crocoite.com |
| [Mandarino |
| Min. Record 24 (1993) | |
| Australian J. Mineralogy 13 (1) | |
| Min. Record 24 (1993) | |
| Grice et al. (1991) | |
| Min.Record 24 (1993) | |
| Ferguson (1999) |
| P. Downes (1998) |
| Hodge (1970) |
| Simpson Mineral Collection of the ... +1 other reference |
| Singer et al. (2008) |
| Simpson Mineral Collection of the ... +2 other references |
| Simpson Mineral Collection of the ... +7 other references | |
| Simpson Mineral Collection of the ... +7 other references | |
| Morris (1961) |
| Simpson Mineral Collection of the ... |
| Fetherston et al. (2013) |
| Downes et al. (2006) |
| Downes et al. (2006) | |
| Murray Thompson |
| Bridge et al. (1979) |
| Downes et al. (2017) |
| Downes et al. (2017) | |
| Simpson Mineral Collection of the ... +1 other reference |
| Clarke et al. (1986) |
| Clarke et al. (1986) | |
| Downes et al. (2011) |
Austria | |
| C.Auer (2014) |
| NIEDERMAYR et al. (2006) |
| C. Auer (2011) |
| A.Pichler (2012) |
| Kolitsch (2009) |
| G. Blass (2001) +1 other reference | |
| Niedermayr et al. (1995) |
| |
| Kolitsch et al. (2013) |
| Niedermayr et al. (1995) |
| Meixner (1976) |
| Blaß et al. (1997) |
| Niedermayr et al. (1995) |
| Chris Auer +1 other reference | |
| Pichler (2009) |
| G. Niedermayr (1998) | |
| F. Brandstätter et al. (1996) | |
| C.Auer (2013) |
| Pichler (2009) |
| Blaß (1999) | |
| Mikl et al. (2014) |
| Niedermayr et al. (1995) |
| Niedermayr et al. (1995) |
| Brandstätter et al. (2011) |
| Steir.Mineralog 25/2011 |
| Puttner (1996) |
| G. Niedermayr: Carinthia II 191./111.:97-102 (2001) |
| Niedermayr et al. (1995) |
| A.Pichler (2012) |
| C.Auer (2013) |
| Niedermayr et al. (1995) |
| Auer (2023) |
| Kolitsch et al. (2014) |
| Kolitsch et al. (2014) |
| C. Auer (2023) |
| Auer (2023) |
| Kirchner et al. (2007) |
| Strasser (1989) |
| Fritz Schreiber collection |
| Kolitsch et al. (2010) |
| Putz (2003) |
| C. Auer: Lapis 20 (11) |
| C. Auer: Lapis 20 (11) | |
| C.Auer (2014) |
| Collection of NHM +1 other reference |
| Brandstätter et al. (2010) |
| Niedermayr (2008) |
| Kolitsch et al. (2009) |
| Kirchner et al. (2004) |
| Strasser (1989) | |
| C. Auer (2022) | |
| Strasser (1989) |
| R.Poeverlein (2016) | |
| Strasser (1989) | |
| R.Poeverlein (2016) | |
| R.Poeverlein (2016) | |
| Strasser (1989) | |
| R.Poeverlein (2016) | |
| Poeverlein (2008) | |
| Strasser (1989) |
| Lewandowski et al. (2006) |
| Schnorrer et al. (2000) |
| C.Auer (1988) |
| Strasser (1989) | |
| Neschen (n.d.) +1 other reference | |
| Kolitsch (2014) | |
| Brandstätter et al. (2010) |
| U. Kolitsch et al. (2012) | |
| Exel (1993) | |
| Landesmuseum Joanneum (Graz, Styria) |
| G.Gesselbauer collection (Find of 2014) |
| Postl et al. (1988) +1 other reference |
| Auer et al. (2017) |
| Gerald Gesselbauer (06.2010) +1 other reference |
| P. Tomazic et al. (2012) |
| Auer (2023) |
| Postl et al. (1996) |
| Kolitsch (2014) |
| Neschen (n.d.) |
| Kolitsch et al. (2011) +1 other reference |
| Neschen (n.d.) |
| Neschen (n.d.) |
| Leikauf (2006) |
| Neschen (n.d.) +4 other references |
| C.Auer (2017) |
| [Lapis 1992: 2 p.19-30] | |
| Neschen (n.d.) |
| Kolitsch (2018) |
| Schachinger et al. (2012) |
| Heider et al. (2019) | |
| Meixner (1976) |
| Gröbner et al. (2007) |
| Meixner (1976) |
| Gröbner (2000) |
| Taucher (1994) |
| Postl (1977) |
| Postl (1977) | |
| Kolitsch (2010) |
| Jakely (2008) |
| Jakely (2008) |
| Jakely (2008) | |
| Gröbner et al. (2012) |
| Bergmann (2011) |
| Steck (2010) |
| Kolitsch (2014) |
| Kolitsch (2014) |
| Poeverlein |
| Schnorrer et al. (2002) |
| Poeverlein et al. (2007) | |
| Der Aufschluß (2006) | |
| Lapis 2007 | |
| Exel (1993) |
| Exel (1993) | |
| Lapis 19 (7/8) | |
| Schnorrer et al. (2010) |
| Schnorrer et al. (2005) | |
| Schnorrer+Poeverlein (2005) | |
| C.Auer (2016) | |
| Kolitsch (2013) | |
| 58. +1 other reference |
| Putz et al. (2007) |
| 54 (in German) +1 other reference |
| 54 (in German) +1 other reference | |
| C.Auer (2015) |
| Arlt et al. (1994) |
| Schnorrer et al. (2007) |
| Schuster et al. (2066) |
| Kolitsch (2013) |
| Kolitsch (2022) |
| Kolitsch (2019) |
| Kolitsch (2014) |
| Kolitsch et al. (2011) |
| Kolitsch et al. (2022) | |
| Gröbner et al. (2007) |
Belgium | |
| Ottenburgs et al. (1998) |
| Hatert et al. (2002) |
| Hatert et al. (2002) | |
| Hatert et al. (2014) |
| van Tassel et al. (1979) +1 other reference |
| Arnold Van Herreweghe Collection |
| José Dehove collection |
| Michel Blondieau. +3 other references | |
| Fransolet et al. (1975) +1 other reference |
| Dehove et al. (2006) |
| Hatert et al. (2002) | |
| Blondieau (2005) | |
| Blondieau (2005) | |
| van Tassel. R. (1979) |
| Hatert et al. (2002) |
Bolivia | |
| Federico Ahlfeld and Jorge Muñoz Reyes (1955) |
| www.mineralmundi.com |
| J. T. Singewald Jr. & Edward Berry (1922) |
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) | |
| USGS Bulletin # 1975 +1 other reference |
| Mineralogical Record: 32: 474. +1 other reference |
Brazil | |
| Cook (2009) |
| USGS Prof Paper 550C ppC190-C196 |
| Pires et al. (2020) |
| Cornejo et al. (2009) |
Bulgaria | |
| Kunov et al. (2001) |
| Larisa Nesheva (2006) |
| Singer et al. (2008) +1 other reference |
| |
| ATANASSOVA +1 other reference |
| Marcus Vau Collection |
Canada | |
| Peatfield (n.d.) +1 other reference |
| JAMBOR (1976) |
| Singer et al. (2008) |
| MINFILE No 104A 008 |
| Woodside (n.d.) +2 other references |
| Phillips (1979) |
| Norman Wilson collection |
| Sabina (2003) |
| Sabina (2003) | |
| SABINA (1976) | |
| Norman Wilson collection |
| Steve Stuart Collection |
| Sabina (1965) |
| Sabina (1965) |
| nsminerals.atspace.com (2005) |
| Collected by Ronnie Van Dommelen (~1999) |
| Sabina (2015) +1 other reference |
| Ann P.Sabina (1991) |
| Sabina (1991) |
| Sabina (1991) |
| Sabina (1991) | |
| Sabina (1991) | |
| Sabina |
| Sabina (2000) |
| Jambor (1967b) |
| Ontario Dept.of Mines |
| Ontario Ministry of Northern ... | |
| Ref.: Geological Survey of Canada ... |
| Sabina (1991) |
| Sabina (1991) |
| Sabina (1991) | |
| Sabina (1991) |
| Traill (1983) |
| Traill (1983) |
| Sabina (2000) | |
| Sabina (2003) +1 other reference |
| Sabina (2003) +1 other reference |
| Sabina (2003) |
| Cobalt - Belleterre - Timmins +1 other reference | |
| HORVÁTH et al. (2006) |
| Sabina (1992) | |
| and parts of New Brunswick. Geological ... +1 other reference |
| Sabina (1992) |
| Sabina (1992) |
| Estrie and Gaspesie +3 other references |
| Sabina (1966) | |
| Sabina (1992) |
| Jennings et al. (1973) |
| Sabina (1983) |
| Sabina (1967 & 1992) |
| HORVÁTH et al. (2000) |
| www.ir.gov.sk.ca (n.d.) |
| Sabina (1972) | |
| Watkinson et al. (1975) |
| Sabina (1972) |
| Dawson Creek +1 other reference |
| Dawson Creek +1 other reference | |
| Singer et al. (2008) |
| Sabina (1972) |
| Sabina (1972) | |
Chile | |
| Arfè et al. (2016) |
| Singer et al. (2008) |
| WARREN (2005) | |
| Econ Geol (1985) | |
| Bárbara Romero et al. (2011) | |
| "Yacimientos costeros entre Antofagasta ... +2 other references |
| Singer et al. (2008) | |
| globenewswire.com (n.d.) |
| Pieczonka et al. (2017) | |
| Sonami: "Boletín de la Sociedad ... +1 other reference | |
| Sonami: "Boletín de la Sociedad ... | |
| |
| Remy Phillippe personal communication | |
| Sernageomin database 2006 +1 other reference | |
| Singer et al. (2008) +1 other reference | |
| Werthessen (2016) |
| British Natural History Museum online ... | |
| V. Sapienza collection |
| Anthony et al. (2000) | |
| Palache et al. (1951) |
| Samples in the I. Domeyko Museum | |
| Palache et al. (1951) +1 other reference |
| Singer et al. (2008) |
| Sapiains et al. (2021) | |
| Singer et al. (2008) | |
| Cameron +2 other references | |
| Maksaev (2006) |
| C. Ruiz Fuller/F. Peebles (1988) | |
| C. Ruiz Fuller/F. Peebles (1988) | |
| C. Ruiz Fuller/F. Peebles (1988) |
| Sernageomin database 2006 +1 other reference |
| JM Johannet collection. |
| Maurizio Dini collection |
| Sernageomin database 2005 +1 other reference | |
| Bob Jenkins & M. Dini collections | |
| M.Dini collection | |
| Williams et al. (2005) |
| Maurizio Dini database 2007 |
| Maurizio Dini database 2007 | |
| Francis et al. (1994) | |
| V. Sapienza collection |
| maurizio dini collection - analysed ... | |
| Palache et al. (1951) | |
| Singer et al. (2008) | |
| mining-atlas.com (n.d.) | |
| collected and analysed by french ... | |
| "Geología del distrito Zapallar de ... +2 other references |
| "Geología del distrito minero Zapallar ... +2 other references | |
| C. Ruiz Fuller/F. Peebles (1988) | |
| Sernageomin database 2006 +1 other reference |
| maurizio dini collection - analysed ... |
| " Geología de los distritos mineros Checo de Cobre (provincia de Atacama) +2 other references | |
| collected and analysed by french ... |
| samples analysed by Gerhard Mohn and ... |
| maurizio dini collection - analysed ... | |
| I.Domeyko Museum collection in the La ... +3 other references |
| samples analysed by Jochen Schlüter +1 other reference |
| collected and analysed by french ... |
| Stgo de Chile. +1 other reference | |
| Arliguie M collection |
| Maurizio Dini collection | |
| Sullivan (1978) | |
| samples analysed by Gerhard Mohn and ... |
| samples analysed by Jochen Schlüter |
| collected and analysed by french ... | |
| Robert Jenkins |
| maurizio dini collection - analysed ... +1 other reference |
| C. Ruiz Fuller/F. Peebles (1988) |
| "Informe preliminar sobre las minas del ... +1 other reference |
| "Informe sobre el mineral de San ... +1 other reference | |
| "Informe preliminar sobre las minas del ... +3 other references | |
| Singer et al. (2008) |
| C. Ruiz Fuller/F. Peebles (1988) |
| Samples in the collection of professor Claudio Canut De Bon (La Serena, Chile) |
| Samples analysed by Dr. Jochen Schluter et al. (Salzburg Department of Mineralogy) |
| Maurizio Dini collection |
| I.Domeyko collection +2 other references |
| Jaime Cataldo B. et al. (2015) | |
| Singer et al. (2008) |
| Samples analysed by Dr. Jochen Schlüter (Hamburg University) |
| Palache et al. (1951) +1 other reference |
| Samples analysed by Dr. Jochen Schlüter (Hamburg University) +1 other reference | |
| Sulphides analysed by geology dep. of ... +2 other references | |
| Palache et al. (1951) |
| U. Kolitsch collection (gift from Arturo Molina) +1 other reference | |
| British Museum online catalogue 2007 |
China | |
| Yi Li (2001) |
| Personally collected David Clayton ... |
| Tony Peterson specimens |
| Jurui He et al. (1992) |
| Jensen (2009) |
| Bert Ottens specimen |
| Wenzhang Fu and Jinchuan Gu (2001) |
| Chunqi Wen et al. (2002) |
| Yi Li et al. (2004) |
| Hercule Shen |
Cuba | |
| Rocks & Min.: 20:169. |
Czech Republic | |
| Velebil (Dě) +4 other references |
| Pauliš P. Mineralogické lokality ... |
| Plášil |
| Plášil J. et al. (2008) | |
| RÜSENBERG et al. (1996) |
| Ondruš et al. (1989) |
| [Bull.N.M.Praha 2002 | |
| [Lapis 1995 |
| Malý |
| Pauliš P. et al. (2015) +1 other reference |
| Lapis 2002 (7/8) | |
| Skala et al. (2011) | |
| |
| František Novák et al. (2002) |
| Beran (Ag, Au, Co) +2 other references | |
| Petr Pauliš (2000) +1 other reference |
| Fengl +3 other references |
| Malec J. et al.: Jacutingait (podkrkonošská pánev) |
| Pauliš et al. (podkrkonošská pánev, Česká republika) | |
| Fojt (A) |
| Sejkora (1994) |
| Kuttna +1 other reference | |
| Sejkora et al. (2023) | |
| Zimák +6 other references |
| Roman Gramblička collection |
| Fiala V. (1977) | |
| Č +4 other references | |
| Sejkora et al. (2012) |
| Roman Gramblička collection |
| none |
| mineral.-petrolog. Odd. Nár. Muz. (Praha) +2 other references | |
| Sejkora (1998) +1 other reference | |
| Vavřinec L. | |
| Sejkora et al. (Krušné hory) +1 other reference |
| |
DR Congo | |
| Paul De Bondt collection. |
| Mineralogical Magazine (1977) | |
| Deliens (1996) | |
| Lapis (1992) | |
| Mineralogical Record: 20: 287-288. |
| Kampunzu et al. (2009) |
| Lapis 17 (3) |
| King (n.d.) |
| Deliens (1996) | |
| Lapis (1992) | |
| Paul De Bondt collection |
| Wilson (2018) |
| Deliens (1989) | |
| |
| Joy Desor (Raman and EDS analyses) |
Egypt | |
| Natural History Museum Vienna (rock) |
| |
Europe | |
| Jansa et al. (Č) +4 other references |
Finland | |
| Helovuori et al. (5) +1 other reference |
| Hytönen |
France | |
| Pierre Le Roch & Jean-Marc Johannet ... +1 other reference |
| Cuchet et al. (2000) | |
| P.G. PELISSON (pelisson@inist.fr) |
| Robin Fialip |
| Belot (1978) |
| Pélisson (1989) |
| C. Vialaron : La mine d'antimoine de Daü et al. (1999) |
| Queneau (n.d.) +1 other reference |
| Roman Gramblička collection |
| Crichton et al. (2008) |
| Designolle J-L. (1993) |
| Chollet et al. (2013) |
| Vernay (1997) +1 other reference | |
| Chollet Pascal Collection |
| Coueille (1988) |
| Paul Poulain | |
| Favreau G. et al. (1996) |
| Valverde J. (1999) |
| Vessely Collection | |
| Sarp et al. (1980) |
| De Ascençao Guedes et al. (2000) |
| De Ascencao Guedes R. (2001) |
| Wittern et al. (Cologne) |
| Chollet Pascal Collection +1 other reference |
| Germain C. et al. (1990) |
| R.De ASCENÇAO GUEDES (2004) |
| Wittern et al. (Cologne) |
| A. Wittern et al. (Cologne) |
| Wittern et al. (Cologne) |
| Th. Brunsperger Collection |
| Wittern et al. (Cologne) |
| Wittern et al. (Cologne) | |
| P&T N°23-24 et al. (Haut-Rhin) | |
| Paolo Grosso collection | |
| R. Stein collection | |
| Wittern |
| Hohl: "Minéraux et Mines du Massif Vosgien" (Mulhouse) |
| Wittern et al. (Cologne) |
| André Marent collection |
| Mines |
| Mines | |
| A. Wittern & J.-R. Journée: "Mineralien finden in den Vogesen" (Cologne) | |
| |
| Pierres et Terre |
| Wittern et al. (Cologne) | |
| Wittern et al. (Cologne) |
| ESCANDE et al. (1973) +6 other references |
| ESCANDE et al. (1973) +6 other references | |
| Dana 6:399 |
| Cédrick Gineste 01.04.2005. | |
| Queneau (n.d.) |
| Queneau (n.d.) |
| Queneau (n.d.) |
| Remy Ph. (2003) |
| Berbain et al. (2000) |
| Forner et al. (1997) |
| Pélisson et al. (1987) |
| Berbain et al. (2005) |
| Bortolozzi (n.d.) |
| idem (2005...) +1 other reference | |
| Berbain et al. (1998) |
| Favreau et al. (2003) | |
| Berbain et al. (2005) |
| Gol (2015) |
| Queneau (n.d.) +1 other reference |
| Lheur (2023) |
| Pierrot +1 other reference | |
| Self - collected by : Jean Marie LAURENT |
| Leconte J. et al. (2016) | |
| Pierrot | |
| Gayraud et al. (2011) +1 other reference |
| Jean-Maire Laurent collection |
| Jean-Marie LAURENT collection | |
| Favreau et al. (2010) |
| Queneau (n.d.) | |
| Queneau (n.d.) |
| Inventaire Minéralogique de la France ... +1 other reference | |
| Jean-Marie LAURENT collection |
| Queneau (n.d.) |
| Le Cahier des micromonteurs |
| Georges Favreau collection |
| Y. Vessely collection |
| Yannick Vessely collection |
| JM Johannet. |
| Le Règne Minéral 47: 5-21 |
| Gourault C. (1997) | |
| collection bernard bouissac/antoine ... |
| Dincuff (2008) |
| Gol et al. (2010) | |
| Boisson et al. (2014) |
| No References | |
| Georges Favreau collection |
| Jean-Marie LAURENT collection |
| D. Gol (2009) |
| Queneau (n.d.) |
| BERBAIN et al. (2010) |
| Le Cahier des Micromonteurs |
| erbain +2 other references | |
| Berbain et al. (2005) |
| Berbain et al. (2005) |
| Queneau (n.d.) |
| Laurent J-M. (2003) |
| Queneau (n.d.) | |
| R.Pauvert |
| desescaut.jy@voila.fr |
| Bernadi et al. (2014) |
| Jean-Vincent Coureau collection |
| Fourcault (1993) |
| Chollet Pascal Collection | |
| Mari et al. (1997) |
| R. Pierrot | |
| R. Pierrot |
| my collection |
| Belot (1978) |
| R. Pierrot | |
| R. Pierrot | |
| R. Pierrot |
| Jean-Luc Portes Collection |
| Pierrot et al. (1972) +1 other reference |
| Pierrot et al. (1972) +1 other reference |
| Self collected by S. MAURY |
| Jean-Marie Claude collection +4 other references |
Germany | |
| Walenta (1992) |
| Emser Hefte 15 (3) |
| Walenta (1992) |
| Markl (2017) | |
| Weiß (1990) |
| Weiß (1990) | |
| Baret et al. (1986) |
| Baret et al. (1986) | |
| Walenta (1992) | |
| Weiß (1990) | |
| Walenta (1992) |
| Gruber (2000) | |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) | |
| Lapis 21 (12) |
| Lapis 21 (12) |
| www.mineralienatlas.de (2021) | |
| Walenta (1992) | |
| Wittern (1995) |
| Anneliese Schwarz collection (visually identified by Uwe Kolitsch) +1 other reference | |
| Blaß et al. (2000) |
| Wittern (2001) |
| Schrenk (2000) |
| Walenta (1992) |
| Walenta et al. (1984) +1 other reference |
| |
| Lapis 21 (12) |
| Wittern (2001) |
| Walenta (1992) |
| Gröbner (2007) |
| Weiß (1990) |
| |
| |
| Walenta (1992) | |
| [Lapis 1992 |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| Walenta (1992) |
| A. Wittern (1995) |
| 54. +1 other reference |
| American Mineralogist: 67: 854. +1 other reference |
| Walenta (1992) |
| Walenta (1992) |
| Wittern (2001) |
| Walenta (1992) |
| Wittern (2001) | |
| Lapis (2) |
| Habel (2009) |
| Wittern (2001) |
| Lapis 32 (12) |
| |
| Hock et al. (1992) |
| Wittern (2001) |
| 54. +1 other reference |
| Lapis 2002 (11) |
| 54. +1 other reference |
| www.berthold-weber.de (2001) |
| Dill et al. (2010) | |
| PETITJEAN collection |
| ex-Petitjean collection |
| Collection of Steffen Michalski |
| Lapis 25 (2) | |
| Belendorff et al. (1987) | |
| Belendorff (2021) |
| Klaus Petitjean collection |
| Blessing et al. (1991) |
| Stephan Koch photo |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| SEM-EDS by Günter Blaß |
| Blaß et al. (1993) |
| Weiß (1990) |
| Weiß (1990) | |
| Joachim Gröbner (2019) |
| Weiß (1990) |
| Schnorrer et al. (2009) |
| Weiß (1990) | |
| Fehr (1983) | |
| Neschen (n.d.) |
| Wittern et al. (1986) +1 other reference |
| ko Jansen. +1 other reference | |
| "Lithothek" collection of the ... | |
| Schnorrer-Köhler (1991) | |
| Nikoleizig et al. (2010) | |
| Gröbner et al. (2011) | |
| Gröbner et al. (2009) | |
| Gröbner et al. (2011) |
| |
| Gröbner et al. (2011) | |
| |
| Luetcke (n.d.) |
| |
| Neschen (n.d.) | |
| van den Berg et al. (1990) |
| Neschen (n.d.) | |
| Luetcke (n.d.) |
| Gröbner et al. (2011) |
| Luetcke (n.d.) | |
| Gröbner et al. (2011) |
| J. Gröbner: Neufunde aus den Bergbaurevieren St. Andreasberg et al. (2007) |
| |
| Hotze (1989) | |
| Gröbner et al. (2011) |
| Schnorrer et al. (2001) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| |
| Schnorrer (1995) +1 other reference | |
| Schnorrer-Köhler (1991) |
| Lapis (2) |
| Schnorrer-Köhler (1986) +1 other reference |
| Mineralien-Welt 19 (1) |
| Wittern (2001) |
| Weiß (1990) |
| Weiß (1990) |
| Wittern (2001) +1 other reference |
| Mills et al. (2020) | |
| Wittern (2001) |
| Rolf Golze +3 other references |
| Reinhardt et al. (2016) | |
| Wittern (2001) |
| Lapis (12) |
| Schnorrer-Köhler (1987) |
| Hucko et al. (2009) |
| Rolf Golze +3 other references |
| Blaß et al. (1995) |
| Weiß (1990) |
| Blaß et al. (1995) |
| Blaß et al. (1995) | |
| Blaß et al. (1995) | |
| Graf et al. (1982) |
| GeoMontanus: Die Mineralien von Rescheid |
| GeoMontanus: Die Mineralien von Rescheid |
| Blaß et al. (1995) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) | |
| min max |
| Remmert (2023) |
| Weiß (1990) |
| Lapis 1988 (1) |
| Nino Martincevic Collection |
| Lapis 1988 (1) | |
| Lapis 1988 (1) | |
| Lapis 1988 (1) |
| Weiß (1990) |
| Volker Reppke Collection |
| Schüller et al. (2013) |
| 54. +1 other reference |
| Volker Reppke Collection |
| Wittern (2001) |
| Emser Hefte 1990 (1) |
| Weiß (1990) |
| Emser Hefte 1987 (4) |
| Weiß (1990) |
| Henrich (2007) | |
| Günter Frenz collection +1 other reference |
| www.mgas.de (2003) |
| Christof Schäfer |
| Scheiderhöhn & Kautzsch 1936 |
| Lapis (2) |
| Lapis 2001 (6) |
| Lapis (5) |
| Lapis (5) | |
| Wittern (2001) | |
| Wittern (2001) | |
| Graf et al. (1991) |
| Thomas Kleser Collection |
| Markl et al. (1990) |
| Lapis (3) |
| Weiss: "Mineralienfundstellen et al. (Munich) |
| Habel (2006) +1 other reference | |
| Wittern (2001) |
| |
| Wittern (2001) |
| Weiß (1990) |
| 58 (in German) +1 other reference |
| Schnorrer-Köhler et al. (1991) | |
| Weiß (1990) |
| Weiß (1990) |
| Weiß (1990) |
| Vanrusselt (2023) |
| Weiß (1990) |
| Gröbner et al. (2011) |
| Gröbner et al. (2011) |
| Gerfried Seidel collection |
| Thomas Lühr |
| Gröbner et al. (2011) |
| Gröbner et al. (2011) |
| Wittern (2001) | |
| Thomas Lühr Collection | |
| C.Auer (2019) |
| C.Auer (2019) |
| Neschen (n.d.) |
| Neschen (n.d.) |
| Gröbner et al. (2011) |
| Gröbner et al. (2011) |
| Wittern (2001) | |
| Wittern (2001) |
| Wittern (2001) |
| Möhn et al. (07/2020) |
| Vollstädt et al. (1991) |
| Massanek et al. (2005) |
| Lapis (10) | |
| www.mineralienatlas.de (n.d.) |
| Schlegel (1991) |
| Gröbner J. et al. (2006) |
| www.mineralienatlas.de (2016) |
| Hajek (2010) |
| www.dergraul.de (2001) |
| 86. +1 other reference |
| Gröbner J. et al. (2006) |
| Wittern (2001) |
| Wittern (2001) |
| Mineralien-Welt (2) |
| Wittern (2001) |
| Wittern (2001) |
| Tröger (2012) |
| Matthies (2009) |
| Markus Gerstmann - Collection |
| 43-47 +2 other references |
| T. Witzke & F. Rüger: Lapis 1998 (7/8) |
| T. Witzke & F. Rüger: Lapis 1998 (7/8) |
| 54. +1 other reference |
| J. Gröbner & J. Wesiger (2008) |
| T. Witzke et al.: Lapis 2001 (12) |
| Wittern (2001) |
| F. Rüger (1) |
| Wittern (2001) |
| Rüger et al. (1992) | |
Greece | |
| Busz (1893) +2 other references |
| Gröbner et al. (2002) | |
| Hanke (1998) | |
| LAPIS 24 (7/8) +1 other reference | |
| Gröbner (2001) | |
| Schnorrer (1995) +1 other reference |
| Lapis et al. (1999) |
| Branko Rieck collection |
| Lapis (May 2003) | |
| Gröbner (2003) | |
| Blaß et al. (1998) |
| |
| Lapis et al. (1999) +1 other reference |
| Fritz Schreiber collection +1 other reference |
| Fritz Schreiber collection | |
| |
| Lapis et al. (1999) | |
| Lapis et al. (1999) | |
| Lapis 24 (1999) |
| Branko Rieck collection |
| Blaß et al. (1998) | |
| Lapis et al. (1999) +1 other reference | |
| Branko Rieck collection |
| Schnorrer-Köhler et al. (1988) | |
| Fritz Schreiber collection | |
| www.brgm.fr (2003) |
| Rocks and Minerals (2009) |
| |
| Keil et al. (1982) |
| Fritz Schreiber collection (analysed by U. Kolitsch) |
| Keil et al. (1982) | |
Greenland | |
| Petersen (2001) |
Hungary | |
| Szakáll et al. (1996) |
| Szakáll: Minerals of Rudabánya +2 other references |
| Coll. G.Koller |
| Tamás Ungvári +1 other reference |
| GEODA 2013/2 |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. | |
| ACTA MIN. PETR. Suppl. Tomus XXXVIII. +1 other reference | |
| Szakáll et al. (1996) | |
| Csanad Loranth (XRD analysis) |
Iceland | |
| Sigurdur H. Markússon +1 other reference |
India | |
| S. G. Tenainkai et al. (1991) |
| Panigrahi et al. (1997) |
Indonesia | |
| Maryono et al. (2018) |
| Singer et al. (2008) |
Iran | |
| Sadati et al. (2016) |
| Nezafati et al. (2017) |
| K. J. Henley (1970) +1 other reference |
| Vachik Hairapetian collection |
| Mohsen Mohammadi |
Ireland | |
| S. Moreton collection |
| Dr Richard Unitt Collection |
| Moreton et al. (2007) |
| Moreton et al. (2007) | |
| |
| |
| Cornwall |
| Dr Richard Unitt Collection |
| Dr Richard Unitt Collection |
| Moreton et al. (1995) |
| Moreton et al. (1995) |
| Ixer |
| S. MORETON et al. (1999) | |
| |
| Moreton (2005) |
Isle of Man | |
| Adam Stephens Collection |
Israel | |
| Bartura et al. (1974) |
Italy | |
| Toffolo (2012-13) |
| • Barresi et al. (2005) |
| Tumiati et al. (2005) |
| Larocca (2012) |
| Demartin et al. (2014) +1 other reference |
| Russo et al. (2014) | |
| Russo et al. (2014) |
| Dalrio (1980) |
| ADORNI F. (1997) |
| Adorni F. (1997) | |
| Adorni F. (1997) | |
| Aspetti mineralogici dei "Gessi ... |
| Bortolozzi et al. (2017) |
| Bortolozzi (n.d.) +1 other reference | |
| EDX analysis by Italo Campostrini |
| Bortolozzi et al. (2015) |
| Ciuffardi et al. (2008) |
| Biagioni et al. (2020) |
| M.E. Ciriotti et al. (P. Rögner analysis) |
| Rivista Mineralogica Italiana (Avril/Juin) |
| Redazionale (2005) |
| Pipino (1984) |
| Camarda et al. (2013) | |
| Balestra (2016) |
| Agliuzza et al. (2015) |
| Carbone et al. (2002) |
| M.E. Ciriotti Probed 2005 |
| Bracco (2006) |
| G.M.Gramaccioli |
| Capitanio A. (2000) |
| Luigi Possenti Collection |
| Maida et al. (2012) | |
| Bortolozzi (n.d.) |
| Cerea et al. (2019) |
| Rivista Mineralogica Italiana |
| Vergani et al. (2021) |
| Vergani et al. (2020) | |
| Carmagnola et al. (2019) |
| Vergani (2019) +1 other reference |
| Vergani et al. (2020) | |
| Vergani et al. (2020) | |
| Vergani F. et al. (2020) |
| Bedognè et al. (1993) |
| AA. VV. |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) |
| Biffi et al. (2004) +1 other reference |
| Cingolani G. (1984) |
| EDX + XRD analysis by Gunter Blass |
| Sergio Varvello collection |
| Piccoli et al. (2007) |
| Piccoli et al. (2007) |
| Maletto et al. (1976) +1 other reference |
| Ciriotti et al. (2019) |
| Ciriotti et al. (2019) | |
| Ciriotti et al. (2019) | |
| Piccoli et al. (2007) |
| Guastoni et al. (2006) +2 other references |
| Guastoni A. et al. (2006) +2 other references | |
| Albertini (1991) +2 other references | |
| Luigi Chiappino data |
| Bortolozzi (n.d.) |
| Fernando Caboni et al. (2023) |
| Del Caldo et al. (1973) +1 other reference |
| Gamboni et al. (2021) |
| Olmi F. et al. (SS) |
| Olmi F. et al. (1995) | |
| Stara et al. (1996) |
| Stara P. (1996) |
| Stara et al. (1996) | |
| Moldovan et al. (2013) | |
| Stara | |
| Cavinato A. (1939) +1 other reference | |
| Stara et al. (1996) |
| Stara et al. (1996) | |
| Brizzi G. et al. (1989) | |
| Stara et al. (1996) | |
| Pflügel (1982) | |
| Stara et al. (1996) | |
| Fernando Caboni et al. (2024) | |
| Cadoni et al. (1) +1 other reference | |
| Brizzi et al. (1994) |
| Stara P. (1996) | |
| Preite et al. (2007) |
| Stara |
| Stara et al. (1996) | |
| Stara et al. (1993) |
| Lovisato (1908) +3 other references |
| www.associazionemineralogicasarda.it (n.d.) | |
| Rivista Mineralogica Italiana 3/1992-"I minerali del giacimento di Monte Tamara (Nuxis) |
| Stara et al. (1993) |
| Stara et al. (1993) | |
| Stara et al. (1996) |
| [AmMin 85:1563] +2 other references |
| Bortolozzi (n.d.) |
| Mair (1996) |
| Bortolozzi (n.d.) +1 other reference | |
| Ferretti et al. (2017) |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) | |
| Conedera M. et al. (2016) |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) | |
| Bortolozzi G.M. et al. (2022) |
| Maiello et al. (2009) +1 other reference |
| Bortolozzi et al. (2021) |
| Rocchetti I. (1992) |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) | |
| Bortolozzi et al. (2013) |
| Campostrini et al. (2005) |
| Bortolozzi (n.d.) |
| Paolo Gasparetto et al. (2014) | |
| Bortolozzi (n.d.) | |
| Fabio Tosato +3 other references | |
| Ferretti et al. (2019) |
| "Lithothek der Münchener Micromounter" ... |
| Bardi et al. (2017) +1 other reference |
| Sabelli C. et al. (GR) | |
| Bazzoni et al. (2007) | |
| "Lithothek der Münchener Micromounter" ... |
| Jansen et al. (1998) | |
| Batacchi et al. (2013) |
| Bazzoni et al. (2001) | |
| Biagioni et al. (2013) |
| Int. Assoc. of Collectors of Slag Minerals (2) | |
| www.comune.pisa.it (2000) | |
| Perchiazzi et al. (1990) | |
| Am Min 67:385-393 +1 other reference | |
| Orlandi et al. (1985) |
| W. Strohschneider (1975) |
| Libvrna N°5 - 2022 |
| Bonifazi (2021) |
| Franzini et al. (1992) +2 other references |
| P. Rögner et al. (2000) |
| Jansen et al. (1998) |
| Luigi Chiappino data |
| Biagioni (2009) |
| |
| Biagioni (2009) |
| Capperi M. et al. (FI) |
| Brizzi et al. (3) |
| Nannoni R. |
| Bortolozzi (n.d.) |
| Bortolozzi (n.d.) |
| A. Perugini collection |
| |
| Sergio Pegoraro et al. (2022) +2 other references |
| Lapis (2) +1 other reference |
| Saccardo D. et al. (Torrebelvicino, Vicenza) |
| Lapis +1 other reference |
Japan | |
| Suzuki et al. (1976) +1 other reference |
| Sadanaga et al. (1974) +1 other reference |
| Dr. Kameki Kinoshita collection (curated at Geological Survey of Japan) |
| Sadanaga et al. (1974) |
| Matsubara (2008) |
| Mineralogical Journal Vol. 18 (1996) |
| Minerals in Japan (Field Best Encyclopedia V. 15, 2003) |
| Excelibur Mineral Co. specimen |
| - (Tada-ginzan article in the show guide for the 5th Osaka mineral show) +1 other reference |
| Yamada (2004) |
| Yamada (2004) |
| Yamada (2004) |
| |
| Uehara et al. (2014) |
| Minerals in Japan (Field Best Encyclopedia, vol. 15) |
| Kusachi et al (1986) |
| Munich Lithothek specimens (2015 Versandliste) |
| Yamada (2004) |
| Ohnishi et al. (2001) |
| Yamada (2004) |
| Sadanaga et al. (1974) +1 other reference |
| Yamada (2004) | |
| Akira Kato (2009) |
| Shirose et al. (2011) +1 other reference |
Kazakhstan | |
| Kalinin et al. (2018) |
| Pavel M. Kartashov analythical data of ... |
| Litvinovich et al. (1960) |
| Jeremolenko (2002) |
| maurice.strahlen.org (2016) |
| Evseev (1995) | |
| Singer et al. (2008) |
| maurice.strahlen.org (2003) |
Kyrgyzstan | |
| Pekov (1998) |
Laos | |
| Michael Stott |
Luxembourg | |
| Philippo et al. (2007) |
| Philippo Simon (éditeur) | |
Madagascar | |
| Lacroix (1899) |
| Behier (1963) |
| - (n.d.) +1 other reference | |
Mexico | |
| Swoboda (1998) |
| Wilson (1980) |
| Panczner (1987) |
| Querol +1 other reference |
| Hoffmann (1968) +1 other reference |
| Thomas P. Moore (2008) | |
| Panczner (1987) | |
| Panczner (1987) |
| Panczner (1987) | |
| Panczner (1987) |
| |
| Copper Handbook 1911 |
| Espinosa Perea (1999) |
| Panczner (1987) |
| Lapis 2001 (1) |
| Thorne (n.d.) | |
| Valencia et al. (2006) +2 other references |
| Panczner (1987) |
| |
Mongolia | |
| Singer et al. (2008) |
Morocco | |
| Georges Favreau collection |
| Fabre (n.d.) +1 other reference |
| Giovanni Scapin Collection |
| Georges Favreau collection |
| Georges Favreau collection | |
| Georges Favreau collection | |
| Georges FAVREAU collection + EDS | |
| JP Barral collection | |
| Georges Favreau collection | |
| Favreau et al. (2006) |
| [MinRec 18:160 |
Mozambique | |
| Paul Van Hee photo and specimen |
Myanmar | |
| Dill et al. (2013) |
Namibia | |
| von Bezing (2007) |
| Moore (2010) |
| Niedermayr (2001) |
| Thorne (n.d.) |
| Mineralogical Record. Tsumeb: 8: 20 +1 other reference |
| von Bezing (2007) |
| Francois Retief collection |
| von Bezing (2007) |
| von Bezing (2007) +1 other reference |
New Zealand | |
| Railton et al. (1990) |
| a new occurrence. Mineralogical Magazine +1 other reference |
| Essence of Microscope 2012 | |
| Railton et al. (1990) | |
| Courtney et al. (1990) |
Norway | |
| Breivik (2015) |
| Raade (1972) +2 other references |
| Juul Nilsen (1993) | |
| Knut Edvard Larsen collection # MM-6590 | |
| Juul Nilsen (1993) | |
| Nordrum (1995) |
| Raade (1972) |
| Raade (1995) | |
| |
| Neumann (1985) +1 other reference |
| Neumann (1985) |
| Garmo (1974) |
| Neumann (1985) | |
| Garmo (2013) | |
| Husdal (2021) |
| Michalsen (2019) |
| Neumann (1985) |
| Knut Eldjarn photo & specimen |
| Witsø (1995) |
| Witsø (1999) +1 other reference |
| Witsø (1995) | |
| Geological Survey of Norway. The Ore ... | |
| Atle Michaelsen collection & photo (From Torgeir Garmo) |
| Nordrum (2003) |
| Neumann (1985) |
| Eldjarn (1977) |
| Raade (1973) +1 other reference | |
| Folvik (2005) | |
| Berg (1994) |
Oman | |
| Pracejus et al. (2017) |
Pakistan | |
| Ikram Mineralogy | |
| Ikram Mineralogy |
Peru | |
| Singer et al. (2008) |
| Singer et al. (2008) |
| Singer et al. (2008) | |
| Petersen (1970) |
| Singer et al. (2008) |
| Singer et al. (2008) |
| Singer et al. (2008) | |
| Petersen (1970) |
| Hyrsl et al. (2003) +1 other reference |
| Hyršl (2011) |
| Hyršl (2011) | |
| Hulse (2015) |
| Singer et al. (2008) |
| Singer et al. (2008) | |
Philippines | |
| Natural History Museum Vienna ore ... |
Poland | |
| Ref.: Gołębiowska B. et al. (near Cracow, S Poland) |
| Siuda (2004) |
| Siuda (2001) | |
| Mochnacka et al. (2012) | |
| Ciesielczuk +5 other references |
| Siuda (2011) |
| Eligiusz Szełęg collection (SEM/EDS identification) |
| Mederski et al. (2021) |
| Wieser et al. (1986) |
Portugal | |
| Marques de Sá et al. (2010) |
| Alves (n.d.) |
| Field observations (Pedro Alves, 2017) |
| Alves (n.d.) |
| Pedro Alves analytical data |
| Alves (n.d.) |
| Pedro Alves collection and analytical ... |
| Pedro Alves collection and analytical ... | |
| Pedro Alves analytical data and ... |
| Pedro Alves collection and analytical ... |
| Pedro Alves collection and analytical ... | |
| Pedro Alves collection and analytical ... |
| Pedro Alves collection and analytical ... |
| Pedro Alves collection and analytical ... |
| Alves (n.d.) |
| Martins da Pedra collection |
| SXRD-analysed by Dr. Uwe Kolitsch |
| Pedro Alves collection and analytical ... |
| Hugo Lobo Collection |
| Elmar Lackner specimen (single-crystal XRD analysis by Uwe Kolitsch, August 2006) |
| Schnorrer-Köhler (1991) |
| Pedro Alves collection and analytical ... | |
| Alves (n.d.) |
| Rui Nunes collection |
| Alves (n.d.) | |
| Alves (n.d.) |
| Alves (2017) |
| |
| Alves et al. (2017) |
| |
| Pedro Alves collection et al. (2002) |
| Pedro Alves (2013) |
Romania | |
| G.Koller 2008-2009 |
| Palache et al. (1951) |
| J Behier collection |
| minerals-of-the-carpathians.eu (2008) | |
| Szakáll (2002) | |
| Acta Mineralogica Petrographica |
| Apopei et al. (2005) |
| Andrei I. Apopei et al. (2014) | |
| Ł. Kruszewski visual identification |
Russia | |
| Pekov I.V. et al. (2010) +1 other reference |
| Pekov et al. (2011) |
| Cesnokov et al. (1998) |
| Nikolaev et al. (2013) +1 other reference |
| Pekov (1998) |
| Pekov et al. (2022) |
| Bortnikova et al. (2017) |
| PEKOV et al. (2013) |
| Mikhailova et al. (2007) | |
| Arzamastsev et al. (2008) | |
| Ivanyuk et al. (2018) |
| Mikhailova et al. (2015) | |
| Grant et al. (2001) |
| Pavel M. Kartashov analytical data (2012) |
| Mikhailov et al. (2021) |
| Palache et al. (1951) +1 other reference |
| Kievlenko E.V. (1983) | |
| Palache et al. (1951) |
| Kasatkin et al. (2021) |
| NJMM (1993) +1 other reference | |
| Kuzhuget et al. (2015) |
| Palyanova et al. (2018) |
| Gongalsky et al. (2019) |
| Rob Lavinsky specimen +6 other references |
Slovakia | |
| Kodě +3 other references |
| Kodě +2 other references | |
| Števko et al. (2016) |
| Števko M. | |
| Koděra (1986) |
| Martin Števko-unpublished | |
| Koděra (1986) |
| Števko M. et al. (2018) |
| Hoppanová et al. (2023) |
| Koděra (1986) |
| Grecula (1995) |
| Martin Števko-unpublished |
| Števko M. (2014) |
| Vlasáč et al. (2021) |
| Ozdín D. & Gregor M. |
| Sejkora J. |
| Háber et al. (SGR) | |
| Pauliš P. | |
| Martin Števko-unpublished | |
| Koděra (1986) | |
| Števko M. (2022) | |
| Koděra (1986) |
| Martin Števko-unpublished |
| Koděra (1986) | |
| Martin Števko |
| Martin Števko-unpublished | |
| Števko M. (2022) | |
| Martin Števko-unpublished |
| Pauliš P. |
| Koděra (1986) |
| Koděra (1986) |
| Koděra (1986) | |
| Koděra (1986) | |
| Chovan et al. (1995) |
South Africa | |
| S. Weinert (2006) |
| Robert O. Meyer collection +1 other reference |
| Atanasova et al. (2016) |
| Meulenbeld et al. (2014) |
| Cairncross et al. (1995) |
| SAMS (South African Micromount Society) |
| SAMS (South African Micromount Society) | |
| Atanasova et al. (2016) |
| Wight (2002) |
| Cairncross et al. (1995) |
| Meyer et al. (1986) | |
| Schoch et al. (1985) |
| Cairncross (2004) | |
| Steffen Michalski Collection - all ... |
Spain | |
| Georges Favreau collection |
| Ko Jansen | |
| Calvo Rebollar et al. (2022) |
| Calvo Rebollar (2014) |
| Valladares et al. (2021) | |
| Valladares et al. (2021) |
| in Spanish version (Revista de Minerales) +1 other reference |
| A. Arribas et al. (2005) |
| Christiane & Jean-Robert Eytier ... |
| Marcel Valladares collection |
| Rewitzer et al. (part 2) |
| Carmona Ruiz et al. (2018) |
| MineralUp (Revista de Minerales) +1 other reference |
| César Menor Salván et al. (2014) |
| Palache et al. (1951) |
| Calvo (2018) |
| Centro de interpretación de la ... |
| Calvo (2008) |
| Calvo (2014) |
| Calvo (2014) |
| www.foro-minerales.com (2022) |
| www.foro-minerales.com (2022) +1 other reference | |
| Mineralogistes de Catalunya (2/3) |
| Ignacio Herrera +13 other references |
| Joan Rosell |
| Pedro Mingueza et al. (2022) |
| Rodríguez +4 other references |
| Joan Abella i Creus and Joan Viñals i Olià (2012) +1 other reference |
| Calvo (1994) |
| Joan Abella i Creus (2008) |
| Minerals as determined by analysis by ... +1 other reference |
| José González del Tánago. "Minerales ... |
| Calvo Rebollar (2014) |
| MinRec-2003-0708-315 |
| Fabre (n.d.) |
Sweden | |
| Jonsson (2010) |
| Persson (2006) |
| Öhman et al. (2004) |
Switzerland | |
| |
| Meisser (1999) +1 other reference |
| EDXS analyses | |
| Meisser (1999) | |
| Romani (2000) +1 other reference |
| Die Mineralien der Schweiz +1 other reference |
| Schweizer Strahler |
| Stalder et al. (1998) |
| Gröbner J. (2017) |
| Stobbe (1987) |
| Stalder et al. (1998) |
| Stalder et al. (1998) |
| Cuchet et al. (2012) |
| |
| Kolitsch (1998) | |
| Ansermet (2012) |
| EDXS analyses +1 other reference |
| Stalder et al. (1998) |
| Stalder et al. (1998) |
| Meisser (2012) |
| Stalder et al. (1998) |
| Stalder et al. (1998) +1 other reference | |
| EDXS analyses | |
| Stalder et al. (1998) | |
| Ansermet (2012) | |
| Ansermet (2012) |
| Ansermet (2012) |
| Ansermet (2012) |
| Ansermet (2012) |
| Ansermet (2012) |
| Stalder et al. (1998) +1 other reference | |
| Ansermet (2012) | |
| 86. (in German) +2 other references | |
| Stalder et al. (1998) +1 other reference | |
| Ansermet et al. (2021) | |
| Stalder et al. (1998) +1 other reference | |
| Personally collected by Günter Frenz |
Taiwan | |
| Singer et al. (2008) |
Tajikistan | |
| Badalov et al. (1975) |
| Turlychkin et al. (1972) | |
| Pekov (1998) |
Tunisia | |
| Garnit et al. (2018) |
Turkey | |
| Yigit (2012) |
| Kines (1969) |
| Kines (1969) | |
UK | |
| |
| Day (1999) |
| Semmons (1885) +1 other reference | |
| Knight et al. (1970) +1 other reference |
| Collection Richard De Nul |
| Richard de Nul collection | |
| Peter Trebilcock. | |
| Braithwaite et al. (1982) |
| |
| Virginia Maine collection | |
| Virginia Maine collection |
| P Haas (2007) | |
| Dale Foster / Crofty Consultancy - ... | |
| Bruce et al. (1998) |
| |
| Martin Stolworthy Collection | |
| |
| LeBoutillier et al. (2003) | |
| BMS Newsletter 79 (http://britishmicromountsociety.homestead.com/Gannell-Smelter.html) |
| Golley et al. (1995) |
| S. Rust collection. | |
| Specimen from Dragon Minerals |
| G.Curtis collection |
| LeBoutillier et al. (2000) |
| |
| Peter Trebilcock.Self Collected. |
| Elton et al. (2004) |
| Golley et al. (1995) |
| Dines (1956) |
| Rudler (1905) |
| Chris Popham Collection | |
| Paul De Bondt collection |
| Jay I.G. Roland |
| Paul De Bondt collection |
| Richard de Nul collection |
| collected in April 2003 by P Haas +1 other reference |
| Ian Jones collection |
| Ansermet (2007) |
| Paul De Bondt collection |
| |
| Collection Richard De Nul |
| Virginia Maine collection |
| Virginia Maine collection | |
| Robin Selley and Roger Eslick (2004) |
| Miers (1894) +1 other reference | |
| Steve Rust collection |
| Golley et al. (1995) +1 other reference | |
| P Haas (collected 2007) |
| Richard de Nul collection | |
| S. Rust collection | |
| Paul De Bondt collection |
| Golley et al. (1995) |
| P Haas | |
| Golley et al. (1995) |
| Steve Rust collection |
| Elton (1998) |
| Embrey et al. (1987) |
| Golley et al. (1995) |
| Norman Wilson collection |
| Jarvis +2 other references |
| Day (1999) |
| S. Rust collection | |
| Kingsbury et al. (MS) +3 other references |
| Cooper et al. (1990) | |
| Kingsbury et al. (MS) +3 other references | |
| Davidson et al. (1951) +4 other references |
| Mike Leppington collection (visual ID) | |
| - (2006) |
| Hartley (1984) +2 other references | |
| Davidson et al. (1951) +2 other references | |
| Kingsbury (xxxx) +4 other references |
| Davidson et al. (1951) +2 other references | |
| comprising a familiar account of ... +12 other references | |
| Cooper et al. (1990) | |
| Day (1999) | |
| Paul Nicholson Collection |
| Paul Nicholson |
| Stanley et al. (1991) | |
| Day (1999) |
| BMS Collection |
| |
| Green et al. (2000) |
| University Manchester Museum | |
| NHM (2010) |
| Kingsbury et al. (MS) +2 other references |
| Kingsbury (xxxx) +1 other reference |
| Briscoe et al. (2008) | |
| |
| Day (1999) |
| Braithwaite (1983) +1 other reference |
| Braithwaite (1983) | |
| Ford et al. (1993) | |
| Render (n.d.) |
| Render (n.d.) | |
| S. Rust collection |
| Chris Popham |
| Render (n.d.) |
| Render (n.d.) |
| Virginia Maine collection |
| Barry Pitt personally collected from the waste dumps in 2022 Specimen A – Sky blue material. XRD no. NMW X-3884 = probable cyanotrichite (far from a perfect match) | |
| |
| Conroy (2023) | |
| Braithwaite et al. (1982) | |
| |
| Rumsey et al. (2008) |
| Hubbard et al. (2005) |
| Day (1999) |
| Render (n.d.) |
| Braithwaite (1982) |
| Render (n.d.) |
| Render (n.d.) |
| Render (n.d.) |
| Steve Rust collection |
| Rust (1995) |
| Day (1999) | |
| Render (n.d.) |
| Wet chemical identification by Dr ... |
| Livingstone et al. (1976) +2 other references |
| Braithwaite et al. (1990) | |
| Anthony (1997) | |
| Day (1999) |
| Day (1999) |
| Green (1987) +2 other references |
| S. Rust collection | |
| S. Rust collection. | |
| Day (1999) | |
| S.Rust Collection | |
| Render (n.d.) |
| Render (n.d.) | |
| |
| Braithwaite (1982) | |
| Steve Rust collection |
| Bevins (1994) |
| Day (1999) |
| Rust et al. (1988) | |
| S Rust collection | |
| |
| S Rust collection |
| Mason et al. (1996) |
| S. Rust collection |
| S.Rust Collection |
| S.Rust collection |
| Chris Popham Collection | |
| Day (1999) | |
| Jones (1987) | |
| |
| M.M.Wirth collection |
| Green et al. (1996) +1 other reference |
| |
| Day (1999) | |
| |
| Mason et al. (1997) |
| Bevins et al. (1985) |
| Day (1999) |
| Dave Evans collection |
| S. Rust collection |
| |
| S.Rust Collection |
| Day (1999) |
| Cotterell et al. (2011) |
| Render (n.d.) |
| Rust et al. (1987) | |
| Render (n.d.) | |
| Render (n.d.) |
| S. Rust collection | |
| Render (n.d.) | |
| Render (n.d.) | |
Ukraine | |
| A. Tischenko. Minerals of the Crimea - ... |
| Dvoichenko (1914) +1 other reference |
USA | |
| Rocks & Min 70:5 pp 320-333 |
| - (2008) |
| Ransome (1904a) +2 other references |
| Ransome (1904) +7 other references |
| Richard Graeme | |
| Graeme (1993) +1 other reference | |
| Luetcke (n.d.) |
| Rob Bowell | |
| Robert Bowell and Rolf Luetcke | |
| Singer et al. (2005) | |
| Ransome (1904) +5 other references |
| Galbraith (1959) +1 other reference | |
| Luetcke (n.d.) |
| "The Accidental Pocket" talk presented ... |
| Ron Layton self collected | |
| Bob Jenkins |
| Luetcke (n.d.) |
| Luetcke (n.d.) |
| Luetcke (n.d.) |
| Luetcke (n.d.) |
| Luetcke (n.d.) | |
| Luetcke (n.d.) |
| Luetcke (n.d.) |
| Bruce J. Murphy | |
| Galbraith (1959) | |
| Luetcke (n.d.) | |
| Thorne (n.d.) | |
| Rob Bowell | |
| Rob Bowell | |
| Marek Chorazewicz (2023) |
| Butler et al. (1938b) +2 other references |
| Luetcke (n.d.) |
| Rolf Luetcke | |
| Anthony et al. (1995) | |
| Anthony et al. (1995) | |
| Leicht (1971) +1 other reference |
| Wenrich et al. (1992) |
| Gornitz (2004) |
| MRDS database Dep. ID #10026861 +1 other reference |
| - (2005) | |
| Kiersch (1949) +3 other references |
| Eastlick |
| Bideaux et al. (1960) +1 other reference |
| Frank Karasti |
| MRDS database Dep. ID #10027438 |
| Jones (1980) +2 other references |
| MRDS database Dep. ID #10027008 |
| Hutton (1959b) +1 other reference |
| - (2005) | |
| Robinson et al. (1966) +1 other reference |
| Dana 7:II:578 +1 other reference |
| Dana 6: 945 | |
| Univ. of AZ Bull. 41 (1916-17) | |
| Lindgren (1904) +6 other references | |
| Univ. AZ Bull. 41 (1916-17) |
| Univ. AZ Bull. 41 (1916-17) | |
| MRDS database Dep. ID #10088902 |
| MRDS database Dep. ID #10088900 | |
| MRDS database Dep. ID #10282444 +1 other reference | |
| MRDS database Dep. ID #10048285 |
| MRDS database Dep. ID #10048130 |
| MRDS database Dep. ID file #10027704 |
| Galbraith (1959) +1 other reference |
| Anthony et al. (1995) |
| Williams et al. (1975) +1 other reference | |
| Keith (1978) +1 other reference |
| Luetcke (n.d.) | |
| Anthony et al. (1995) |
| John Lucking |
| MinRec 19 (3) | |
| Verfied by Bob Jenkins |
| Genth (1868) +2 other references |
| Hill (1914b) +2 other references |
| Granger et al. (1962) +1 other reference |
| Reinhardt (1952) |
| Gilluly (1937) +3 other references |
| Williams et al. (1963) +1 other reference |
| Williams (1963) +1 other reference | |
| Ronald Render |
| Luetcke (n.d.) |
| Anthony et al. (1995) |
| Singer et al. (2008) |
| Anthony et al. (1995) |
| Anthony et al. (1995) | |
| Anthony et al. (1995) |
| Galbraith (1959) |
| Anthony et al. (1995) |
| Khin (1970) +1 other reference |
| Mineralogy of Arizona |
| Anthony et al. (1995) |
| Galbraith (1959) |
| Anthony et al. (1995) |
| 297-309. +3 other references |
| MRDS database Dep. ID #10096172 |
| MRDS database Dep. ID #10186598 | |
| Palache et al. (1951) +1 other reference |
| MRDS database Dep. ID #10048300 | |
| Jones (1983) |
| Anthony et al. (1995) |
| Barnes et al. (1983) |
| Anthony et al. (1995) |
| Dana 6: 1094 +3 other references | |
| Luetcke (n.d.) |
| Wilson (1933) +1 other reference |
| Rocks & Min.: 63:122 +1 other reference |
| Rogers (1912) +1 other reference |
| Pemberton (1983) |
| Van Nostrand Reinholt Press: 297. +1 other reference |
| Van Nostrand Reinholt Press: 297. +2 other references |
| Van Nostrand Reinholt Press: 297 +3 other references |
| Van Nostrand Reinholt Press: 297 +4 other references |
| Rocks & Min.: 23:504-507. |
| Mineral News: 16 (9) +3 other references | |
| with a section on the stratigraphy of ... +7 other references |
| Paul M. Adams (2003) |
| Kampf et al. (2016) +1 other reference |
| Mineral News Vol 16-5 Adams +1 other reference |
| Robert M. Housley (2005) |
| Robert M. Housley (2005) | |
| Ball (1907) +1 other reference |
| Dr. Robert Housley |
| MarekC pers. coll. 2017-2018 |
| Dunning et al. (2005) |
| Dunning et al. (2005) | |
| Dunning et al. (2005) | |
| Van Nostrand Reinholt Press: 298. +2 other references |
| Daniel J. Evanich visual identification |
| Joseph F. Cooper Jr. et al. (2003) +1 other reference |
| Bowen et al. (1954) +1 other reference |
| Van Nostrand Reinholt Press: 226 +2 other references |
| Van Nostrand Reinholt Press: 298. +2 other references | |
| [Mineralien Welt 1/93:44] |
| Luetcke (n.d.) | |
| Murdoch (1966) | |
| Housley et al. (1999) |
| Luetcke (n.d.) +1 other reference |
| Van Nostrand Reinholt Press: 298. +2 other references |
| Kampf et al. (2010) |
| Thorne (n.d.) | |
| Joe Marty specimens | |
| Thorne (n.d.) | |
| Murdoch (1966) | |
| Crowley (1977) +3 other references |
| Collected by and in the collection of ... | |
| Collected by and in the collection of ... | |
| Tony Kampf (PXRD analysis) |
| Palache et al. (1951) |
| Eckel et al. (1997) |
| - (2005) |
| - (2005) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Eckhard D. Stuart collection (XRD by Canadian Museum of Nature) |
| Eckel et al. (1997) |
| Beroni +2 other references |
| Rocks & Minerals 82:368-380 |
| Eckel et al. (1997) | |
| Rocks & Minerals 81:356-361 |
| Eckel et al. (1997) |
| Eckel et al. (1997) |
| Rocks & Minerals 80:5 pp358-359 |
| Kampf et al. (2019) |
| Thorne (n.d.) | |
| In the collection of Alex Earl | |
| Jeremy Zolan Collecton- Specimens ... |
| Cook (1978) |
| Ream (2004) |
| Ream (2004) |
| Ream (2004) | |
| Ream (1995) |
| - (2005) |
| - (2005) |
| Chris DeGrave Collection |
| Ream (1995) |
| Ream (2004) | |
| Ream (2004) | |
| Ream (2005) |
| Ream (2004) |
| Ream (1995) |
| Ream (2004) |
| King et al. (1994) |
| |
| King et al. (1994) |
| King et al. (1994) |
| Mines of the Washington D.C Area |
| Bernstein (1980) |
| Dunn et al. (1975) |
| Tom Rosemeyer collection |
| Heinrich et al. (2004) +1 other reference |
| Heinrich et al. (2004) |
| Sherwood et al. (1998) |
| Sherwood et al. (1998) |
| Sherwood et al. (1998) |
| www.mineralnews.com (2002) |
| Thorne (n.d.) |
| Gobla (2012) |
| Min News 21:7 p 1 | |
| The Mineralogy of The Butte District +2 other references |
| Castor et al. (2004) |
| Castor et al. (2004) +1 other reference | |
| - (2005) |
| Castor et al. (2004) +1 other reference | |
| Rocks & Minerals |
| Castor et al. (2004) +1 other reference | |
| Luetcke (n.d.) |
| - (2005) |
| Schilling (1979) +2 other references |
| Castor et al. (2004) |
| LaPointe et al. (199) |
| Rocks & Minerals |
| Castor et al. (2004) | |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Jensen et al. (1995) |
| Castor et al. (2004) |
| Castor et al. (2004) | |
| ncma.minresco.com (n.d.) |
| Dr. William S. Wise presentation to ... |
| Collection of Alex Earl | |
| USGS Prof Paper 798D +1 other reference |
| - (2005) | |
| Singer et al. (2005) | |
| Castor et al. (2004) | |
| Castor et al. (2004) |
| - (2005) |
| - (2005) | |
| American Mineralogist 10:137-139 |
| Rocks & Minerals |
| - (2005) |
| Castor et al. (2004) | |
| Adolph Knopf (1918) +1 other reference |
| from Jungles collection | |
| Rocks & Minerals |
| Pullman | |
| Castor et al. (2004) |
| Rocks & Minerals |
| Tony Nikischer |
| Walstrom (n.d.) |
| Castor et al. (2004) | |
| The Minerals of Rhyolite Pass |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) | |
| Doebrich +2 other references | |
| Ross (1961) +1 other reference | |
| Ross (1961) |
| U.S.G.S. Bulletin 973E |
| Castor et al. (2004) | |
| Ross (1961) |
| Canadian Mineralogist Vol. 31 |
| Castor et al. (1993) | |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) +1 other reference | |
| Peggy L. Smith (1983) |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Ameican Mineralogist +2 other references |
| Jensen (1993) | |
| Luetcke (n.d.) | |
| Luetcke (n.d.) |
| Luetcke (n.d.) |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) |
| Castor et al. (2004) | |
| Castor et al. (2004) | |
| Am Min 57:3 pp 364-367 +1 other reference |
| Smith |
| A. Plante collection +3 other references |
| Woodward (1944) |
| Minerals of Laurel Hill et al. (published privately) |
| Sassen (1978) |
| Cook (1973) +2 other references |
| Glover et al. (1975) +1 other reference |
| Northrop et al. (1996) | |
| Glover et al. (1975) +1 other reference | |
| - (2005) +1 other reference |
| Walstrom (n.d.) |
| Northrop et al. (1996) | |
| Northrop et al. (1996) | |
| 2008 New Mexico Mineral Symposium ... |
| 2008 New Mexico Mineral Symposium ... +1 other reference | |
| Luetcke (n.d.) |
| Chester S. Lemanski |
| Walstrom (n.d.) | |
| Walstrom (n.d.) | |
| Walstrom (n.d.) |
| R&M 71:1 p66-68 +1 other reference |
| Robert E. Wastrom Collection |
| Walstrom (n.d.) | |
| Northrop et al. (1996) |
| Northrop et al. (1996) |
| Northrop et al. (1996) | |
| R & J DeMark Minerals of the Carnahan ... | |
| R&M 67:6 p390-395 | |
| Henry L. Jicha (1954) +1 other reference |
| Northrop et al. (1996) |
| Northrop et al. (1996) | |
| Collected by Ray DeMark |
| Northrop et al. (1996) | |
| Northrop et al. (1996) |
| Rocks & Minerals: 78 (6) | |
| Ian Merkel | |
| Michael C Michayluk Collection | |
| John S Sobolewski collection | |
| Mineralization of the Hansonburg Mining ... | |
| M Massis collection |
| [Mineralogical Record 30:337] | |
| Northrop et al. (1996) | |
| Collection of Patrick Haynes. Found in ... | |
| Januzzi +1 other reference |
| Goble et al. (1980) |
| Chamberlain et al. (2001) |
| Carpenter III (1976) |
| - (2005) |
| Carpenter III (1976) |
| - (2005) | |
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) | |
| Jason B. Smith collection |
| Rocks & Minerals 72:4 pp 252-264 |
| Lobell (1986) | |
| - (2005) | |
| Rocks & Min. vol. 72 (1997) |
| The Mineralogy of Pennsylvania 1966-1975 +1 other reference |
| Smith et al. (1977) |
| Sloto (1989) |
| Sloto (1989) |
| Lapham & Geyer |
| Smith et al. (1984) |
| Smith and Hioff (1984) | |
| Smith (1978) |
| Smith (1991) |
| Smith (1991) | |
| Smith (1991) |
| Smith (1991) | |
| Smith (1991) | |
| Price et al. (1985) | |
| R&M 68:6 p381-393 |
| Collection of Alex Earl |
| |
| - (2005) |
| R&M 68:6 p381-393 | |
| Butler (1913) +2 other references |
| Chris Tucker Collection |
| Butler (1913) | |
| Collected by and in the collection of ... | |
| - (2005) |
| USGS: Geological Survey Circular 312 +2 other references |
| |
| Page et al. (1956) +3 other references |
| Page et al. (1956) +3 other references | |
| - (2005) |
| Bullock (1981) |
| Coolbaugh et al. (2020) |
| Roberts et al. (1997) |
| Bullock (1981) | |
| Bullock (1981) |
| Thorne (n.d.) | |
| Dana 6: 1092. +3 other references | |
| Collected by and in the collection of ... | |
| Thorne (n.d.) | |
| Bullock (1981) |
| Thorne (n.d.) |
| Thorne (n.d.) |
| Finnell et al. (1963) |
| Bullock (1981) | |
| Kampf et al. (2008) |
| Joe Marty Collection |
| University of Utah XRD Report 01/25/16 |
| Roland Schmidt Collection No. 2321 | |
| - (2005) |
| Kampf et al. (2015) |
| Patrick Haynes. IDs by the late Howard ... | |
| Kampf et al. (2018) | |
| Bullock (1981) | |
| - (2005) |
| Plášil et al. (2013) |
| Rick Dalrymple Collection | |
| Luetcke (n.d.) | |
| USGS TEI #514 +5 other references | |
| - (2005) | |
| Mandarino (1999) | |
| Bullock (1981) |
| Bullock (1981) | |
| Kokinos et al. (1993) |
| Kokinos et al. (1993) | |
| Kokinos et al. (1993) | |
| Bullock (1981) |
| Thorne (n.d.) +1 other reference |
| Rocks & Minerals 83:1 pp 52-62 |
| Bullock (1981) |
| Palache et al. (1951) +1 other reference |
| Bullock (1981) |
| - (2005) |
| Dietrich (1990) |
| Dietrich (1990) |
| Dietrich (1990) | |
| Dietrich (1990) |
| Dietrich (1990) | |
| Dietrich (1990) |
| Singer et al. (2008) |
| Washington Geology |
| Loucks (1976) |
| - (2005) | |
Zambia | |
| personal visits and collecting in 1996 ... |
Zimbabwe | |
| Vetter et al. (1999) +1 other reference |
Quick NavTopAbout BrochantiteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence SynonymsOther LanguagesCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List
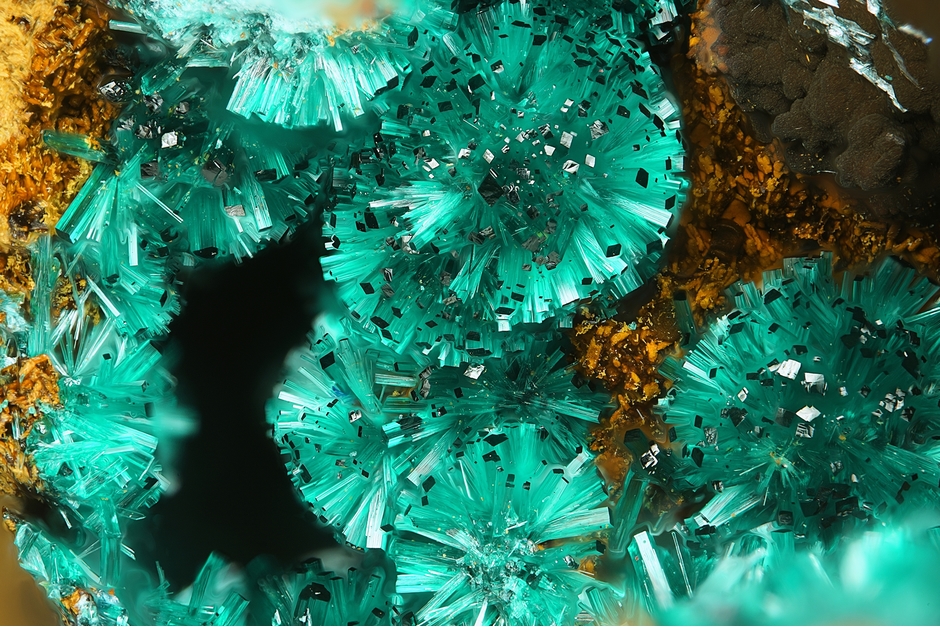






 symbol to view information about a locality.
The
symbol to view information about a locality.
The 





Jean Baptiste Mine, Kamariza Mines, Agios Konstantinos, Lavrion mining district, Lavreotiki, East Attica, Attica, Greece