|
Afghanistan | |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
- Lal Wa Sarjangal District
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Jose Zendrera Collection |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
| Orris et al. (2002) |
|
Albania | |
|
| Buletini i Shkencave Gjeologjike 2012 ... |
|
Algeria | |
|
| Rocks & Minerals (1991) |
|
Angola | |
|
| Milesi et al. (2006) |
|
Antarctica | |
| |
|
| Bell (1984) |
|
| Vennum +1 other reference |
|
Argentina | |
|
| Milka K. de Brodtkorb (2002) |
|
| - (n.d.) |
- Antofagasta de la Sierra department
| Singer et al. (2008) |
|
| FOGLIATA |
|
| Padrón de Minas de la Provincia de Chubut (2005) |
|
| LANFRANCHINI |
- Ciénaga del Coro District
| Sureda (1978) |
|
| Dina (1993) |
|
| Nelson Valenzuela. |
|
| Singer et al. (2005) |
|
| Milka K. de Brodtkorb (2002) |
|
| 4 Congreso Nacional y 1 Congreso ... +1 other reference |
|
| Raúl Jorge Tauber Larry |
|
| Blasón et al. (Ed. E. O. Zappettini) |
- Coronel Felipe Varela department
| A. Brodtkorb y M. K. de Brodtkorb (1999) |
- General Lamadrid Department
| Grundmann et al. (2018) |
|
| de Brodtkorb +2 other references |
|
| Bordese et al. (2016) |
|
| Milka K. de Brodtkorb (2002) |
|
| Singer et al. (2008) |
|
| Grundmann et al. (2018) |
|
| Palache et al. (1951) |
|
| Grundmann et al. (2018) |
- Pampa Amarilla mining district
| American Mineralogist: 51: 1-13 |
|
| KOSTADINOFF |
|
| Philip Persson specimen |
|
| PONS |
|
| Singer et al. (2008) |
|
| Raul Jorge Tauber Larry specimen |
|
| C. Lemanski (re-entry of lost Mindat data) |
|
| Herrmann et al. (2007) |
- Veinticinco de Mayo Department
| American Mineralogist (1978) +1 other reference |
|
| Brodtkorb (1963) |
|
| A. G. Quiroga y T. Ruíz (1994) |
- San Antonio de los Cobres
| Singer et al. (2008) +1 other reference |
|
| www.aldebaranresources.com (n.d.) |
|
| Brodtkorb (2002) |
|
| Collection of Juan Carlos Lodovichi |
- Coronel Pringles Department
| Nilda E. Urbina & P. Sruoga (2008) |
|
| Nilda E. Urbina & P. Sruoga (2008) |
|
| - (2009) |
|
| Singer et al. (2008) |
- Lago Buenos Aires Department
| Permuy Vidal et al. (2016) |
|
| Páez et al. (2016) |
|
| Peña (1970) +1 other reference |
|
| Raúl Jorge Tauber Larry |
|
| Hugo A. Peña (1970) |
|
| Raúl Jorge Tauber Larry |
|
Armenia | |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Khachaturian (1958) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
Australia | |
- Australian Capital Territory
| |
|
| NSW Geological Survey: MR 00762 +1 other reference |
|
| Brown R.E. (1997) |
|
| R.E Brown and W.J Stroud |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Hertzberger et al. (1978) |
|
| Hertzberger et al. (1978) |
|
| Hertzberger et al. (1978) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Vera Munro-Smith (2006) |
|
| Stevens (1972) |
|
| Singer et al. (2005) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Bowman et al. (1976) |
|
| Michael Hirst Collection |
|
| Hertzberger et al. (1978) |
|
| Dieter Mylius collection |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| McKinnon et al. (2005) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Degeling (1982) |
|
| Degeling (1982) |
|
| Wagga Wagga Mine Data Sheets |
|
| Wagga Wagga Mine Data Sheets |
|
| Wagga Wagga Mine Data Sheets |
|
| McQueen et al. (1985) |
|
| Henley et al. (2001) |
|
| Brown et al. (2001) |
|
| Henley et al. (2001) |
|
| Brown et al. (2001) |
|
| Henley et al. (2001) |
|
| R.E Brown and W.J Stroud |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) +1 other reference |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Chrismas et al. (Pty.) +1 other reference |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Degeling (1982) |
|
| Gilligan et al. (1992) |
|
| Gilligan et al. (1992) |
|
| Gilligan et al. (1992) |
|
| B. G. Lottermoser: P. M. Ashley (1997) |
|
| Gilligan et al. (1992) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Gilligan (1975) |
|
| Byrnes (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Singer et al. (2008) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Vera Munro-Smith (2006) |
|
| Henley et al. (2001) +1 other reference |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Gilligan et al. (1992) |
|
| Weber et al. (1972) |
|
| Gilligan et al. (1995) |
|
| Keith F Compton collection (self collected) +1 other reference |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1993) |
|
| Narromine 1:250 000 Mine Data Sheets +1 other reference |
|
| Gilligan et al. (1995) |
|
| Stevens (1972) |
|
| Australian J. Mineralogy 13 (2) |
|
| Stevens (1972) |
|
| Donald H. McColl (1973) +1 other reference |
|
| Singer et al. (2005) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| Henley et al. (2001) |
|
| |
|
| Geological Survey Records +1 other reference |
|
| Henley et al. (2001) |
|
| R.E Brown and W.J Stroud |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) +1 other reference |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Gilligan et al. (1993) |
|
| Graham et al. (2005) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Graham et al. (2005) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Australian Jour. Mineralogy 10:2 pp ... |
|
| A.L. McLean et al. (2004) +1 other reference |
|
| Australian Journal of Mineralogy 10:2 ... +1 other reference |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| NSW Geological Survey report 1970/657 ... |
|
| Felton E. Anne (1975) |
|
| Felton E. Anne (1975) |
|
| Felton E. Anne (1975) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) +1 other reference |
|
| Gilligan et al. (1995) |
|
| Robert Lavinsky specimen +1 other reference |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Gilligan et al. (1993) |
|
| Brown et al. (1997) |
|
| Brown et al. (1997) |
|
| Geochemical et al. (ed.) +1 other reference |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Brown et al. (1992) |
|
| Gilligan (1975) |
|
| Gilligan (1975) |
|
| Gilligan (1975) |
|
| Gilligan (1975) |
|
| Australia and New Zealand Micromineral ... |
|
| Gilligan (1975) |
|
| Gilligan (1975) |
|
| Brown et al. (1992) |
|
| Narromine 1:250 000 Mine Data Sheets |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Leverett et al. (2005) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Leverett et al. (2003) |
|
| Gilligan et al. (1995) |
|
| Leverett et al. (2005) |
|
| Gilligan et al. (1995) |
|
| Gilligan et al. (1995) |
|
| Carne (1908) |
|
| Stevens (1972) |
|
| Geochemical et al. (ed.) +2 other references |
|
| Gilligan et al. (1992) |
|
| Brown et al. (1992) |
|
| McIlveen (1975) |
|
| Gilligan et al. (1992) |
|
| Hertzberger et al. (1978) |
|
| Hertzberger et al. (1978) |
|
| Hertzberger et al. (1978) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Carne (1908) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Department of Mines |
|
| Stevens (1972) |
|
| Stevens (1972) |
|
| Wagga Wagga Mine Data Sheets |
|
| Wagga Wagga Mine Data Sheets |
|
| Birch et al. (1997) |
|
| Keith Compton |
|
| Worner et al. (et al) |
- Broken Hill South Mine (BHS Mine; South Mine)
| Trevor Dart |
|
| Birch et al. (1997) |
|
| Vera Munro-Smith (2006) |
|
| Museum Victoria collection |
|
| Hibbs et al. (2002) |
|
| Stevens B.P.J. et al. (2003) |
|
| Day (1998) |
|
| Barnes (1980) |
|
| The Daydream Mine |
|
| Mines of the Broken Hill District |
|
| Markham et al. (1975) |
|
| Basedow (1924) +2 other references |
|
| Ryan (1961) |
|
| Ryan (1961) |
|
| Ryan (1961) |
|
| Ryan (1961) |
|
| Ryan (1961) |
|
| AMI Resources Pty Ltd (2016) |
|
| Modat Mineral Occurrence database +1 other reference |
- Mosquito Creek Wolfram Field
| Modat |
|
| Cruikshank et al. (1993) |
- Harts Range (Harts Ranges; Hartz Range; Hartz Ranges)
| Skwarnecki (2004) |
|
| Core Exploration Ltd (2015) |
|
| Warren (1980) |
|
| Modat |
|
| Huckitta 1996 |
|
| Zia U. Bajwah |
|
| Huckitta 1996 |
|
| Zia U. Bajwah |
|
| Zia U. Bajwah |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Zia U. Bajwah |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Huckitta 1996 |
|
| Zia U. Bajwah |
|
| Huckitta 1996 |
|
| Wygralak et al. (1998) |
|
| Huckitta 1996 |
|
| Bajwah Z.U. (1996) |
|
| NT Mines + personal collection |
|
| Bajwah Z.U. (1996) |
|
| Bajwah Z.U. (1996) |
|
| Holmes (1972) |
|
| Huckitta 1996 |
- Pinnacles Bore Copper Area
| Shaw (1970) |
|
| Shaw (1970) |
|
| Shaw (1970) |
|
| Shaw (1970) |
|
| Shaw (1970) |
|
| Rohde (2004) |
|
| Rodhe (2005) +1 other reference |
|
| Sorrell (n.d.) +1 other reference |
|
| C J Sullivan and R S Matheson (1952) |
|
| coreexploration.com.au (n.d.) |
|
| Dehne McLaughlin and Ray Grant (2012) +1 other reference |
|
| Ahmad et al. (compilers) |
|
| Ahmad et al. (compilers) |
|
| Ahmad et al. (compilers) |
|
| Adams (1983) |
|
| Modat Mineral Occurrence database +1 other reference |
|
| Modat Mineral Occurrence database +1 other reference |
|
| Modat Mineral Occurrence database +1 other reference |
|
| Modat Mineral Occurrence database +1 other reference |
|
| Lohan (1990) |
- Redbank Area breccia pipes
| www.portergeo.com.au and ... |
|
| |
|
| |
|
| Con Constantinides collection |
|
| Sielecki (1988) |
|
| Keith F Compton collection |
|
| Day et al. (1996) |
|
| de Keyser (1958) |
|
| Sielecki (1988) |
|
| Anon. et al. (1) |
|
| Per Vic Cloete. |
|
| Vera Munro-Smith (2006) +1 other reference |
|
| Day (1998) +1 other reference |
|
| Vera Munro-Smith (2006) |
|
| Australian Journal of Mineralogy Volume ... |
|
| Lawrence et al (1998) |
|
| Per Vic Cloete. |
|
| self collect.australian museum id +1 other reference |
|
| S Rust collection +1 other reference |
|
| Sorrell (n.d.) |
|
| Vera Munro-Smith (2006) |
|
| Crane et al. (2001) |
|
| Vera Munro-Smith (2006) |
|
| Pat Sutton |
|
| Queensland Government Department of ... |
|
| Queensland Government Department of ... |
|
| Queensland Government Department of ... |
|
| Queensland Government Department of ... |
|
| Day +1 other reference |
|
| Brown et al. (2010) |
|
| Day (1998) |
|
| Harvey (1989) |
|
| Georgetown 1:100 000 Map Sheet |
|
| |
|
| Lehrmann (2012) |
|
| Douglas (2014) +1 other reference |
|
| |
|
| |
|
| Anderson et al. (4) |
|
| Collection of NHM |
|
| Smith +1 other reference |
|
| Updated list originally distributed by ... |
|
| |
|
| Qld Dept of Mines |
|
| Qld Dept of Mines |
|
| Qld mines dept +1 other reference |
|
| Singer et al. (2008) |
|
| Lawrence (2000) |
|
| Willmott et al. (1969) |
|
| Record of mines (1980) |
|
| Record of Mines Summary Card No: 86 |
|
| Record of Mines (1980) |
|
| Record of mines (1980) |
|
| Record of mines (1980) |
|
| Sorrell (n.d.) |
|
| Record of Mines (1980) |
|
| Personal Collection of Mark Willoughby |
|
| Record of mines - Summary card No:25 |
|
| Record of mines - Summary card No:31 |
|
| Record of mines - Summary card No:32 |
|
| Record of mines - Summary card No:37 +1 other reference |
|
| Brown et al. (1908) +1 other reference |
|
| Record of mines - Summary card No:61 |
|
| Noble et al. (1983) |
- Arkaroola Region (Arkaroola Wilderness Sanctuary; Arkaroola Station)
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... |
- Mawson Plateau - Paralana Hot Springs area
| Coats & Blissett (1971) |
|
| Mines Summary Card No:15 |
|
| |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Noble R.J. |
|
| Coats et al. (1971) |
|
| Noble R.J. +1 other reference |
|
| Coats et al. (1971) |
|
| Coats et al. (1971) |
|
| Coats et al. (1971) |
|
| Noble et al. (1983) |
|
| Coats et al. (1971) |
|
| Noble et al. (1983) |
|
| Coats et al. (1971) |
|
| Noble et al. (1983) |
|
| Record of mines (1980) |
|
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... +2 other references |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Brown et al. (1899) +2 other references |
|
| |
|
| H Y L Brown (1893) +1 other reference |
|
| SA Geodata Database - deposit 3212. |
|
| SARIG Mineral Deposit Information |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Record of Mines – Summary Card No:87 |
|
| Brown et al. (1899) |
|
| Brown et al. (1908) |
|
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Barnes (1969) |
|
| Brown et al. (1899) |
|
| Record of mines - Summary card No:297 |
|
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... |
|
| |
|
| Noble R.J. |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
- Vulkathunha - Gammon Ranges National Park
| Noble et al |
|
| Gee +1 other reference |
|
| |
|
| Record of mines - Summary card No:14 +1 other reference |
|
| Record of mines - Summary card No:186 |
|
| Copper Handbook 1911 +2 other references |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Record of Mines Summary Card No:147 |
|
| SA Geodata Database - Mineral Deposit ... |
|
| H Y L Brown (1893) |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Record of mines - Summary card No:219 |
|
| Robertson et al. (1981) |
|
| SA Geodata Database (2016) |
|
| SARIG Mineral Deposit Information +1 other reference |
|
| Record of mines - Summary card No:131 |
|
| Gee et al. (1908) |
|
| Record of mines (1980) |
|
| Record of mines (1980) |
|
| |
|
| Collection of RJ Martin |
|
| Record of mines - Summary card No:91 |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| Brown et al. (1899) |
|
| Record of Mines - Mine Summary Card ... |
|
| C. Lemanski (re-entry lost Mindat data) +1 other reference |
|
| Record Of Mines - Summary Card |
|
| H Y L Brown (1893) |
|
| Record Of Mines - Summary Card |
|
| Noble et al. (1983) +1 other reference |
|
| Am Min 59:307-313 +1 other reference |
|
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... |
|
| |
|
| Record of Mines +2 other references |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Record of Mines Summary Card No:15 +2 other references |
|
| Francis et al. (self published) |
|
| SA Geodata: Record of Mines - Summary ... |
|
| Record of Mines - Summary Card |
|
| Record of Mines - Summary Card |
|
| Minerals from Bundaleer and the ... |
|
| Presonal Collection of M. Willoughby. ... |
|
| Mark Willoughby - Personal Collection |
|
| Noble R.J et al. (1983) |
- South Mt Lofty Ranges (Adelaide Hills)
| Noble R.J et al. (1983) |
|
| Record of Mines-Summary Card No: 40 |
|
| Brown et al. (1908) |
|
| 6627 I +1 other reference |
|
| J.E.Johnson 1981. Mineralogy of the ... |
|
| Sorrell (n.d.) |
|
| SARIG Mineral Deposit |
|
| H Y L Brown (1893) +1 other reference |
|
| Grguric et al. (2017) |
|
| Record of Mines-Summary Card No: 27 |
|
| Brown H.Y.L (1884) |
|
| Record of Mines Summary Card No: 3 |
|
| Gee et al. (1908) |
|
| Record of Mines-Summary Card No: 10 |
- Rapid Bay Limestone Quarry
| Record of mines (1980) |
|
| Drexel et al. (1978) |
|
| Collection of M.Willoughby. |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Record of Mines-Summary Card No: 47 |
|
| Noble |
|
| Mark Willoughby collection |
|
| Record of Mines - Summary Card 83 |
|
| Record of Mines - Summary Card 151 |
|
| Gee +1 other reference |
|
| Griessmann (2011) |
|
| Record of Mines – Summary Card No:156 |
|
| Record of Mines – Summary Card No:32 ... |
|
| Record of Mines – Summary Card No:53 ... |
|
| Brown et al. (1899) |
- Bimbowrie Conservation Park
| C.H.H. Conor (2003) |
|
| Griessmann et al. (2006) |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Gov't Geol. Recon. Map (1954) |
|
| SA Geodata Database - Mineral Deposit ... |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
- Boolcoomatta Reserve (Boolcoomata Station)
| Record of mines of South Australia - 1908 (pers comm. in collection of M. Willoughby.) |
|
| Lapis (2014) |
- Pine Creek - Mutooroo area
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| |
- Mt Victor Plumbago Station
| Campana and king |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| Waterhouse (1971) |
|
| Brown et al. (1908) |
|
| Deposit Number: 1928 +1 other reference |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| HYL Brown |
|
| Record of Mines Summary Card No.232 |
|
| SA Geodata Database - Mineral Deposit ... |
|
| Noble R.J. +1 other reference |
|
| Noble et al. (1983) |
|
| portergeo.com.au (2016) |
|
| Personal Collection of Mark Willoughby |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| Deposit Number: 7301 +1 other reference |
|
| H Y L Brown (1893) +1 other reference |
|
| Deposit Number: 5105 +1 other reference |
|
| SA Geodata Database - Mineral Deposit ... +1 other reference |
|
| Deposit Number: 5150 +1 other reference |
|
| Deposit Number: 5112 +1 other reference |
- Scamander mining district
| Bottrill et al. (2008) +1 other reference |
|
| |
- Central Coast municipality
| www.crocoite.com (2003) |
- Circular Head municipality
| Bottrill et al. (2008) |
- Moina - Middlesex District
| Reid (1919) |
|
| M Latham Collection |
- Waratah-Wynyard municipality
| R Bottrill unpub rept |
|
| Bottrill +1 other reference |
|
| Bottrill |
|
| |
|
| Australian Journal of Mineralogy (2006) |
|
| Judy Rowe |
|
| Edwards et al |
|
| Sorrell (n.d.) |
|
| EDWARDS |
|
| |
|
| Birch et al. (1993) |
|
| Henry et al. (2022) |
|
| Birch et al. (2007) |
|
| Nickel et al. (1993) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Min. Record 24 (1993) |
|
| Min.Record 24 (1993) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Low (1963) |
|
| Simpson (1948) |
|
| Marston (1979) |
- Ullawarra Indigenous Community
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Marston (1979) |
|
| Graham Myerson |
|
| Gibb-Maitland (1908) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Simpson (1948) |
|
| Collins et al. (date?) |
|
| Nickel et al. (1994) +2 other references |
|
| Naldrett (2004) |
|
| Sue Koepke pers. comm. |
|
| Clissold (2007) |
|
| Nickel et al. (1977) |
- Derby-West Kimberley Shire
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Simpson (1948) |
|
| Marston (1979) |
|
| Singer et al. (2008) |
|
| minedex.dmirs.wa.gov.au (2020) +1 other reference |
|
| Marston (1979) |
|
| Gibb-Maitland (1908) |
|
| Gibb-Maitland (1908) |
|
| Copper Mineralisation in Western ... |
|
| Marston (1979) |
|
| Marston (1979) |
|
| Marston (1979) |
|
| Marston (1979) +1 other reference |
|
| Marston (1979) |
|
| Simpson (1948) |
|
| Huston (2007) |
|
| Ferguson (1999) |
|
| Downes et al. (2006) |
|
| Kim McDonald collection |
|
| Blatchford (1912) |
|
| Simpson (1948) |
|
| Copper Mineralisation in Western ... |
|
| Ferguson (1999) |
|
| Woodward (1891) |
|
| Dow et al. (1969) |
- Purnululu National Park (Bungle Bungle)
| www.ga.gov.au (n.d.) |
|
| Booth (1989) |
- Kalgoorlie Consolidated Gold Mines
| Simpson (1948) |
- Siberia Goldfield (Waverley)
| Simpson (1948) |
|
| Simpson (1948) |
- Eulaminna (Murrin Murrin mining centre)
| Ferguson (1999) |
|
| Ferguson (1999) |
|
| Simpson (1948) |
|
| Simpson (1948) |
|
| R Bottrill |
|
| Simpson (1948) |
|
| Ferguson (1999) |
|
| The West Australian newspaper +1 other reference |
|
| www.horseshoemetals.com.au (n.d.) |
|
| Marston (1979) |
|
| Simpson (1948) |
|
| Western Mail newspaper (Perth) |
|
| www.sipa.com.au (n.d.) |
|
| Marston (1979) |
|
| |
- Peak Hill Mining District
| Thompson +1 other reference |
|
| Marston (1979) |
- Yaloginda Goldfield (Bluebird; Eight Mile; Yallowgindat)
| Ferguson (1999) |
|
| Grguric et al. (2006) |
|
| Grguric et al. (2006) |
|
| Bridge et al. (1979) +1 other reference |
|
| Simpson (1948) |
|
| Simpson (1948) |
|
| Simpson (1948) |
|
| www.redstone.com.au (n.d.) |
|
| Downes et al. (2017) |
|
| Simpson (1948) |
|
| Downes et al. (2017) |
|
| Downes et al. (2017) |
|
| Simpson (1948) |
|
| Copper Mineralisation in Western ... |
|
| Maitland (1903) +1 other reference |
|
| Copper Mineralisation in Western ... |
|
| Simpson (1948) |
|
| Ferguson (1999) |
- Karara Conservation Reserve
| Kim Macdonald specimen (local specimen miner) |
|
| visual id-Kim Macdonald |
|
| Simpson (1948) |
|
| Marston (1979) |
|
| Visual ID at site by Kim Macdonald. +1 other reference |
|
| Visual ID specimens Kim ... |
|
| Visual ID by Kim Macdonald at site. +1 other reference |
|
| Simpson (1948) |
|
| Marston (1979) |
|
| Martin et al. (2005) |
|
| Ferguson (1999) |
|
| Martin et al. (2005) |
- Wyndham-East Kimberley Shire
| Ferguson (1999) |
|
| Downes et al. (2011) |
|
| Marston (1979) |
|
| Stephen J Godden (2008) |
|
| Copper Mineralisation in Western ... |
|
| Marston (1979) |
|
| Simpson (1948) |
|
| www.bullseyemining.com.au (n.d.) |
|
| Simpson (1948) |
|
| Abeysinghe et al. (1997) |
|
| www.bullseyemining.com.au (n.d.) |
|
Austria | |
|
| Postl et al. (1991) +1 other reference |
|
| Unger (1967) |
|
| A.Pichler (2003) |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
|
| J. Mörtl: Carinthia II 192./112.:333 (2002) |
|
| M. Puttner: Carinthia II 188./108.:193-199 (1998) |
|
| H. Wiessner: Arch. Vaterländ. Gesch. Topogr. 36./37.:242-243 (1951) |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
- Sankt Veit an der Glan District
| Gröbner (2000) |
|
| G. Blass (2001) |
|
| |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
|
| Auer & Kolitsch (2017) |
|
| H. Meixner: Carinthia II 158./78.:101 (1968) |
|
| Kolitsch et al. (2013) |
|
| Niedermayr et al. (1995) |
|
| |
- Sankt Martin am Silberberg
| Blaß et al. (1999) |
|
| Niedermayr et al. (1995) |
|
| Puttner |
|
| www.indra-g.at (2016) |
- Spittal an der Drau District
| Niedermayr et al. (1995) |
|
| Pichler (2009) |
|
| |
|
| Niedermayr et al. (1995) |
|
| Prasnik (2009) |
|
| Pichler (2009) |
- Heiligenblut am Großglockner
| J. Mörtl: Der Aufschluss 35 (10) |
|
| G. Niedermayr (1998) |
|
| Walter et al. (2021) |
|
| Pichler (2009) |
|
| C.Auer (2024) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| Pichler (2009) |
|
| PICHLER (2009) |
|
| Auer (2023) |
|
| Pichler (2009) +1 other reference |
|
| Pichler (2009) |
|
| H. Meixner (1949) |
- Finkenstein am Faaker See
| Niedermayr et al. (1995) |
|
| G. Blass (2000) |
|
| Puttner (1996) |
|
| M. Puttner: Der Aufschluss 45 (1) |
|
| Niedermayr et al. (1995) |
|
| Niedermayr et al. (1995) |
|
| Brandstätter et al. (2007) |
|
| A.Pichler (2003) |
|
| Carinthia 2012 |
|
| Carinthia II (2013) |
|
| Pichler (2009) |
|
| Niedermayr et al. (1995) |
|
| Hiden (1997) |
|
| |
|
| Hiden (1997) |
|
| Niedermayr et al. (1995) |
|
| G. Niedermayr: Carinthia II 191./111.:97-102 (2001) |
- Rijavitzagraben (Jeravitzagraben; Jeravica; Remscheniggraben)
| Niedermayr et al. (1995) |
|
| G. Niedermayr: Carinthia II 191./111.:97-102 (2001) |
|
| A.Pichler (2012) |
|
| Niedermayr et al. (1995) |
|
| G. Niedermayr: Carinthia II 191./111.:97-102 (2001) |
|
| Meixner (1950) |
|
| O. M. Friedrich: Carinthia II 150./70.:85-104 (1960) |
|
| Niedermayr et al. (1995) |
|
| H. Meixner (1969) |
|
| Mörtl (1985) |
|
| A.Pichler (2012) |
- Bad Sankt Leonhard im Lavanttal
| A.Pichler (2003) |
|
| A.Pichler (2003) |
|
| Brandstätter et al. (2005) |
|
| Natural History Museum Vienna collection (catalogued in 1987) +1 other reference |
|
| Harald Schillhammer collection +1 other reference |
|
| Collection of NHM |
|
| Kolitsch et al. (2017) |
|
| Kolitsch et al. (2013) |
|
| Kolitsch et al. (2011) |
|
| E. Löffler collection +1 other reference |
|
| Gerald Knobloch collection |
|
| [Mineralogical Record 29:184] |
|
| Auer (2004) |
|
| C.Auer (2013) |
|
| Kolitsch et al. (2013) |
|
| Kolitsch et al. (2016) |
|
| Hackenberg 2003 |
|
| Hackenberg 2003 |
|
| Kolitsch et al. (2014) |
|
| Neschen (n.d.) |
|
| Neschen (n.d.) |
|
| Collection of NHM |
- Haidbachgraben (Myrthengraben; Myrtengraben; Heidbachgraben)
| Neuner (1956) |
|
| Huber et al. (1977) |
|
| Hackenberg (2003) |
- Waidhofen an der Thaya District
| - (n.d.) |
- Wiener Neustadt-Land District
| seen at Melk mineral show 3/09 +1 other reference |
|
| Kolitsch et al. (2009) |
|
| Reinhold (1907) |
|
| Strasser (1989) |
|
| Kirchner (1978) +1 other reference |
|
| MEIXNER (1978) +1 other reference |
- St. Johann im Pongau District
| Kolitsch (2010) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| Feitzinger et al. (2009) |
|
| Strasser (1989) |
- Mitterberg mining district
| Fritz Schreiber collection |
|
| Strasser (1989) |
|
| Strasser (1989) |
- Sankt Martin am Tennengebirge
| Strasser (1989) +1 other reference |
|
| Strasser (1989) |
|
| C.Auer (2021) |
|
| Strasser (1989) |
|
| Neschen (n.d.) |
|
| Brandstätter et al. (2013) +1 other reference |
|
| Strasser (1989) |
|
| C. Auer: Lapis 20 (11) |
|
| Strasser (1989) +1 other reference |
|
| Schachinger et al. (2014) |
|
| Neschen (n.d.) |
|
| Collection of NHM +1 other reference |
|
| Brandstätter et al. (2010) |
- Fusch an der Grossglocknerstrasse
| Strasser (1989) |
|
| Strasser (1989) |
|
| Bonifazi Marco +1 other reference |
|
| Strasser (1989) |
|
| Josef Brugger collection |
|
| Neschen (n.d.) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| C. Auer (2022) |
- Schwarzleo mining district
| Strasser (1989) |
|
| R.Poeverlein (2016) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| R.Poeverlein (2016) |
|
| R.Poeverlein (2016) |
|
| Fritz Schreiber collection |
|
| Poeverlein (2008) |
|
| Strasser (1989) |
|
| Strasser (1989) |
- Mittersill Scheelite deposit
| Strasser (1989) |
- Neukirchen am Großvenediger
| Lewandowski et al. (2006) |
|
| Strasser (1989) |
|
| Seemann (1993) |
- Alteck - Hoher Sonnblick area
| Mrazek et al. (1992) |
|
| Strasser (1989) +1 other reference |
|
| Strasser (1989) |
|
| Kutil (2004) |
|
| Strasser (1989) |
|
| Neschen (n.d.) +1 other reference |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| Strasser (1989) |
|
| U. Kolitsch et al. (2012) |
- Bruck-Mürzzuschlag District
| Landesmuseum Joanneum (Graz, Styria) |
|
| Postl et al. (2003) |
|
| Tomazic (2010) |
|
| Hackenberg 2003 |
|
| Hackenberg 2003 |
|
| J.Taucher (2001) +1 other reference |
|
| Aufschluss 1972 (SB) +2 other references |
- Fröschnitz valley (Fröschnitz)
| Hackenberg 2003 |
- Oberdorf (Oberdorf an der Laming)
| Kolitsch (2015) |
|
| Gerald Gesselbauer (2010) |
|
| Gerald Gesselbauer |
|
| G.Gesselbauer (2014) |
|
| Offenbacher (1991) |
|
| Hohl (1929) +1 other reference |
|
| Taucher (1999) |
- Tallak (Dallakkogel; Dallakberg; Tallackkogel; Tallakkogel)
| Bernhard (2010) |
|
| matrixx 5 |
|
| Auer (2023) |
|
| Bojar et al. (1999) |
|
| Aufschluss 1972 (SB) |
|
| FREYN (1902) |
|
| Freyn (1902) |
|
| H.Weninger (1978) |
|
| Postl et al. (1988) |
|
| FREYN (1906) +2 other references |
|
| Leikauf (2006) |
|
| Annalen 1999 +1 other reference |
|
| Leikauf (2003) |
|
| Neschen (n.d.) |
|
| Neschen (n.d.) +2 other references |
|
| Neschen (n.d.) |
|
| www.vstm.at (n.d.) |
|
| [Lapis 1992: 2 p.19-30] |
|
| Neschen (n.d.) |
|
| Postl et al. (1984) |
|
| www.vstm.at (2004) +1 other reference |
|
| Friedrich O. M. (1933) |
|
| Archiv f.Lagerstättenforschung |
|
| |
|
| Mörtl (2005) |
|
| Neschen (n.d.) |
|
| Bergbau in Osttkärnten (Pichler 2003) |
|
| Pichler (2003) |
|
| Neschen (n.d.) |
|
| Raith et al. (2015) |
|
| Gröbner (2000) |
- Weißkirchen in Steiermark
| Postl (1981) |
|
| Moser & Postl (1990) |
- Sankt Martin am Wöllmißberg
| Meixner (1930) |
|
| Kolitsch (2010) |
- Prinzkogel (Prinzenkogel)
| Jakely (2010) |
|
| Jakely (2008) |
|
| Jakely (2008) |
|
| 54. +1 other reference |
|
| 54. +1 other reference |
|
| Steck (2010) |
|
| Lapis 29 (6) |
|
| Gerd Stefanik collection |
|
| Steiner M. et al. (2009) |
|
| Vavtar (1977) +1 other reference |
|
| Meixner (1971) |
|
| Wenger (1977) |
|
| Steiner et al. (2007) |
|
| Vavtar (1977) |
|
| Brandstätter et al. (2010) |
|
| Steiner et al. (2010) |
|
| Poeverlein |
|
| Lapis 19 (7/8) |
- Geyer - Silberberg District
| C.Auer (2013) |
|
| Lapis 19 (7/8) |
|
| Schnorrer et al. (2002) |
|
| Poeverlein et al. (2007) |
|
| Der Aufschluß (2006) |
|
| Lapis 2007 |
|
| Putz et al. (2012) |
|
| Exel (1993) |
|
| Exel (1993) |
|
| Lapis 19 (7/8) |
|
| Lapis 19 (7/8) |
|
| Schnorrer et al. (2010) |
|
| Neschen (n.d.) |
|
| Lapis 19 (7/8) +1 other reference |
|
| Schnorrer+Poeverlein (2005) |
|
| www.vlmf.at (n.d.) |
|
| Lapis 19 (7/8) |
|
| 58. +1 other reference |
|
| Marco E. Ciriotti 15/10/2005 |
- Großkogel (Reither Kogel)
| Neschen (n.d.) |
|
| Lapis 19 (7/8) |
|
| Putz et al. (2007) |
|
| A.Lechner |
|
| Lapis 19 (7/8) |
|
| C.Auer (2021) |
|
| Gerd Stefanik |
|
| Joachim Esche collection |
|
| Lammerer et al. (2011, August) |
- Scheffau am Wilden Kaiser
| Kuntscher (1986) +2 other references |
|
| 54 (in German) +1 other reference |
|
| 54 (in German) +1 other reference |
|
| 54 (in German) +1 other reference |
|
| 58. +2 other references |
|
| Ampferer (1930) |
|
| Reithofer (1939) |
- Komperdell (Komperdell Alp)
| Exel (1982) +1 other reference |
|
| 54. +1 other reference |
|
| 54. +1 other reference |
|
| 54. +1 other reference |
|
| Gstrein (1981) |
- Falkenstein mining district
| |
|
| C.Auer (2015) |
|
| Kostrucha (1988) |
|
| M. Moro collection |
|
| Wenger (1979) |
- Ringenwechsel mining district
| Arlt et al. (1994) |
|
| Lapis 19 (7/8) |
|
| Thilo Arlt Collection |
|
| Schnorrer et al. (2007) |
|
| Schuster et al. (2066) |
|
| Angel et al. (1953) +1 other reference |
- Bad Goisern am Hallstättersee
| Götzinger (1985) +1 other reference |
|
| P.Arthofer et al. (2001) |
- Kirchdorf an der Krems District
| O.Wallenta et al. (1988) |
|
| E.J.Zirkl |
|
| Brandstetter et al. (2020) |
|
| Neschen (n.d.) |
|
| E.Reiter (Oberösterreichs 1930 – 1982) |
|
| Arthofer et al. (2010) |
|
| E.J.Zirkl |
|
| Erich Reiter (Oberösterreich 1930 – 1982) |
|
| Bechter (2010) |
|
| Polz (1989) |
|
| Polz (1989) |
|
| Polz (1989) |
|
| Kolitsch (2015) |
|
| Bechter (2010) |
|
| Exel (1982) |
|
| - (n.d.) +1 other reference |
|
Azerbaijan | |
- Belokan-Avar ore district
| Int J Econ & Environ Geology Vol 1 (2) +1 other reference |
|
| Pavel.M. Kartashov (n.d.) |
|
| Hemon et al. (2012) |
- Nakhchivan Autonomous Republic
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
Bailiwick of Guernsey | |
|
| Henwood (1871) |
|
Belarus | |
|
| Levitskiy et al. (2018, July) |
|
Belgium | |
|
| Hatert et al. (2002) |
|
| Cesàro (1898) +2 other references |
|
| |
|
| www.artistones1.be (n.d.) |
|
| Mélon et al. (1976) +1 other reference |
|
| Buttgenbach (1898) +2 other references |
|
| Hatert et al. (2014) |
|
| Mélon et al. (1976) +1 other reference |
|
| Mélon et al. (1976) +1 other reference |
|
| Cesàro (1898) +3 other references |
|
| Lespineux (1903) +1 other reference |
|
| Lespineux (1903) +1 other reference |
|
| Hatert et al. (2002) |
|
| Hatert et al. (2002) |
|
| Michel Blondieau collection |
|
| Blondieau (1997) +1 other reference |
|
| Calembert (1942) +2 other references |
|
| Mélon et al. (1976) +1 other reference |
|
| Blondieau (2005) |
|
| Forir (1896) +3 other references |
|
Bolivia | |
|
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) +1 other reference |
|
| Petrov (n.d.) |
|
| Salomon Rivas y Federico Ahlfeld (1998) |
|
| U.S. Geological Survey and Servicio Geologico de Bolivia (1991) |
|
| Salomon Rivas & Federico Ahlfeld (1998) |
|
| J. T. Singewald Jr. & Edward Berry (1922) +1 other reference |
|
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) |
|
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) |
|
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) |
|
| Geology and Mineral Resources of the Altiplano and Cordillera Occidental (USGS Bulletin no. 1975) |
|
| Ahlfeld (1955) |
- Ladislao Cabrera Province
| USGS Bulletin # 1975 |
|
| USGS Bulletin # 1975 |
|
| Kempff et al. (La Paz, 2009) |
|
| USGS Bulletin # 1975 (1992) |
- San Cristobal de Lípez District
| USGS Bulletin # 1975 |
|
| Petrov et al. (2006) |
|
| D. Richter (1992) |
|
| D. Richter (1992) |
|
| Kempff et al. (La Paz, 2009) |
|
Bosnia and Herzegovina | |
- Federation of Bosnia and Herzegovina
| Ljudevit Barić |
|
| Rui Nunes collection |
|
| Ljudevit Barić |
|
| - (n.d.) |
|
| Ljudevit Barić |
|
| Veljković (1973) |
|
| Rob Lavinsky. |
|
| Radosavljevic et al. (2005) |
|
Botswana | |
|
| Cairncross (2004) |
|
Brazil | |
|
| USGS Prof Paper 550C ppC190-C196 +1 other reference |
|
| Menezes (n.d.) +1 other reference |
|
| Cook (2002) |
|
| Ricardo Scholz information |
|
| Cornejo et al. (2009) |
|
| Proceedings 30th Intl Geol Cong Vol 16 ... +1 other reference |
|
| Wolfgang Hampel |
|
| Barbour et al. (1979) |
|
| Cornejo et al. (2009) |
|
| Cornejo et al. (2009) |
|
| Pinto V. et al. (2011) |
|
| Cornejo et al. (2009) |
|
Bulgaria | |
|
| Marcus Voigt Collection |
|
| Marcus Voigt Collection +1 other reference |
|
| Marcus Voigt Collection |
- Mineralni Bani Municipality
| Marcus Voigt Collection |
|
| Marcus Voigt Collection |
|
| Marcus Voigt Collection |
- Panagyurishte Municipality
| Marcus Vau Collection |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) +1 other reference |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Lips (2006) |
|
| Marcus Voigt Collection |
|
| Lips (2006) |
|
| Svetoslav Petrussenko et al. (2007) |
|
| Petrov et al. (xxxx) |
|
| Lips (2006) |
|
| Mincheva-Stefanova (1968) |
|
| Marcus Vau Collection |
|
| Neues Jahrbuch für Mineralogie - Monatshefte et al. (10) |
|
| Lips (2006) |
|
| Marcus Vau Collection +1 other reference |
- Balkan Mts (Stara Planina)
| J. Ralph site visit 2023 |
|
| tin.er.usgs.gov (n.d.) +2 other references |
|
| Marcus Vau Collection |
|
| Marcus Voigt Collection |
|
Cambodia | |
|
| Economic and Social Commission for Asia and the Pacific (1993) |
|
Canada | |
|
| Peatfield (n.d.) |
|
| I.C.L. Webster (1992) |
|
| Peatfield (n.d.) +1 other reference |
|
| the world's largest besshi-type ... +1 other reference |
|
| Singer et al. (2008) |
|
| personal correspondence with Giles Peatfield (see notes in description) |
|
| Montgomery (n.d.) |
|
| Montgomery (n.d.) |
- Fort Steele Mining Division
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
- Greenwood Mining Division
| Nixon et al. (2002) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) +1 other reference |
|
| |
|
| Belik (1973) |
|
| Minfile |
|
| Singer et al. (2008) |
|
| ... +1 other reference |
|
| Singer et al. (2008) |
|
| Robert Meyer collection |
|
| Singer et al. (2008) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| MINFILE No 104P 029 |
|
| Singer et al. (2005) |
|
| Singer et al. (2008) |
|
| Sabina (1972) |
|
| Dill et al. (2014) |
|
| R.O. Meyer collection |
|
| Singer et al. (2008) |
|
| Peatfield (n.d.) |
|
| personal correspondence with Giles Peatfield (see comments in description) +1 other reference |
- New Westminster Mining Division
| Peatfield (n.d.) |
|
| Peatfield (n.d.) +1 other reference |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| personal correspondence with Giles Peatfield (see notes in description) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Montgomery (n.d.) |
|
| Peatfield (n.d.) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Richard Gunter Collection |
|
| Dawson |
|
| Peatfield (n.d.) |
|
| Singer et al. (2008) |
- Revelstoke Mining Division
| Peatfield (n.d.) |
- Similkameen Mining Division
| personal correspondence with Giles Peatfield (see comments in description) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| MINFILE No 092K 068 |
|
| correspondance with Giles Peatfied |
- Vancouver Mining Division
| |
|
| Peatfield (n.d.) |
|
| Peatfield (n.d.) |
|
| Cole et al. (part of NTS 63K12) +1 other reference |
|
| Sabina (2015) +1 other reference |
|
| Lines et al. (1996) |
|
| - (n.d.) |
- Newfoundland and Labrador
| Sabina (2003) |
|
| SABINA (1976) |
|
| Norman Wilson collection |
|
| Badham (1975) |
|
| Blackadar (1981) |
|
| Stavinga et al. (2017) |
|
| Northern Mineral Showings Database |
|
| Tyson (1989) |
|
| Lang (1952) |
|
| Rich et al. (1977) |
|
| Coleman (1953) |
|
| R Van Dommelen collection (EDS and chemical analysis) |
|
| Sabina (2015) +1 other reference |
|
| Terry Collett collection |
|
| MacMichael (1975) |
|
| Transactions of the Canadian Institute ... |
|
| Eric Quinter collection |
|
| Felderhof (1978) |
|
| evidence for late incursion of ... +1 other reference |
|
| Dewing et al. (2007) |
|
| MDI Number: MDI31C14SE00146 |
- Centre Hastings Municipality
| Bryan Johnson collection |
|
| Sabina (2007) |
|
| Schroetter |
- Unorganized North Sudbury District
| Mineral Deposit Inventory for Ontario |
|
| Willet Miller (1905) +1 other reference |
|
| Sabina (1991) +1 other reference |
|
| Sabina (1991) |
|
| Reiner Mielke 2014 |
|
| Reiner Mielke |
|
| Reiner Mielke 2015 |
|
| Reiner Mielke 2015 |
|
| Maggie Wilson collection |
|
| Can Mineral December 1971 v. 11 no. 1 ... +1 other reference |
|
| Reiner Mielke |
- Saint-Pierre-de-Broughton (West Broughton)
| HORVÁTH et al. (2006) |
|
| Horvath collection visual id. |
- Gaspésie-Îles-de-la-Madeleine
| Estrie and Gaspesie +3 other references |
|
| Horváth collection specimens |
|
| Girard (1971) |
|
| Sabina (1967 & 1992) |
|
| Fredric A Schuster |
|
| Econ Geol (1994) |
- Les Collines-de-l'Outaouais RCM
- Saint-Pierre-de-Wakefield
| Geological Survey of Canada ... +1 other reference |
|
| Sabina (1972) |
|
| Rich et al. (1977) |
|
| Sabina (1972) |
|
| Dawson Creek +1 other reference |
|
| Dawson Creek +1 other reference |
|
| Jambor et al. (1962) |
|
| Literature references cited |
- Watson Lake mining district
| Sabina (1992) |
- Whitehorse mining district
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Hood (2012) |
|
| data.geology.gov.yk.ca. (2017) |
|
| Tenney et al. (1967-1980) +1 other reference |
|
| Sabina (1972) |
|
| Héon (2004) |
|
| Héon (2004) |
|
| Héon (2004) |
|
| Traill (1970) |
|
| data.geology.gov.yk.ca (n.d.) |
|
| R Tafti |
|
Chile | |
|
| Bárbara Romero et al. (2011) |
|
| Singer et al. (2008) |
|
| Samples analysed by Jochen Schluter (chemical tests for Cu and CO3) |
|
| Cook (1978) |
|
| Sapiains et al. (2021) |
- Cerro Negro mining district
| Maurizio Dini collection |
|
| Sernageomin database 2005 +1 other reference |
|
| M.Dini collection |
- El Salvador mining district
| Maurizio Dini database 2007 |
|
| maurizio dini collection - analysed ... |
|
| checked by my mineral teacher |
- San Pedro de Cachiyuyo mining district
| C. Lemanski collection (7192CL) |
|
| Singer et al. (2008) |
|
| Mr. Terry Szenics. |
|
| letter from CHILE (mr.maurizio dini) |
- Siete Vetas mining district
| Sernageomin database 2006 +1 other reference |
|
| Sernageomin database 2006 +1 other reference |
|
| "Geología del distrito Zapallar de ... +2 other references |
|
| Dini M. +1 other reference |
- Cabeza de Vaca mining district
| Ruiz Fuller C./Peebles F +2 other references |
|
| maurizio dini collection - analysed ... |
|
| " Geología de los distritos mineros Checo de Cobre (provincia de Atacama) +2 other references |
|
| " Geología de los distritos mineros Checo de Cobre (provincia de Atacama) +2 other references |
- Cachiyuyo de Llampos mining district
| Remy Phillippe personal communication |
|
| samples analysed by Gerhard Mohn and ... |
|
| Kampf et al. (2006) |
- Cerro Blanco mining district
| I.Domeyko Museum collection in the La ... +3 other references |
|
| Sociedad Explotadora de Minas" (Copiapó) +1 other reference |
|
| "Informe geológico sobre el distrito ... +2 other references |
|
| Sernageomin database 2006 +1 other reference |
- Pampa Larga mining district
| Samples analysed by Dr. Jochen Schlüter (Hamburg University) |
|
| maurizio dini collection - analysed ... |
|
| "Informe geológico económico sobre el mineral de Las Cañas (Vallenar) +3 other references |
|
| "Informe geológico económico sobre el mineral de Las Cañas (Vallenar) +3 other references |
- San Antonio mining district
| "Informe preliminar sobre las minas del ... +3 other references |
|
| McAllister et al. (1950) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| McAllister et al. (1950) |
- Brillador mining district
| Samples analysed by Dr. Jochen Schluter |
- Arqueros Ag mining district
| Cucurella J. |
|
| maurizio dini collection - confirmed by ... |
- Los Algodones mining district
| C. Ruiz Fuller/F. Peebles (1988) |
|
| Samples in the collection of professor Claudio Canut De Bon (La Serena, Chile) |
- Tambillos mining district
| Pnud Chile/Corfo +3 other references |
|
| Samples analysed by Dr. Jochen Schluter et al. (Salzburg Department of Mineralogy) |
|
| samples analysed by Dr. Hubert Putz |
- Mantos de Punitaqui District
| McAllister et al. (1950) |
|
| Singer et al. (2008) |
- Huantajaya mining district
| Maurizio Dini |
- Collahuasi mining district
| Samples analysed by Dr. Jochen Schlüter (Hamburg University) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
- San Felipe de Aconcagua Province
| M.Dini collection |
|
China | |
|
| Zhilin Chen and Jun Lu (1991) |
|
| Rob Lavinsky specimens |
|
| Singer et al. (2008) |
|
| Jiajing Zhang et al. (2008) |
|
| Bingbin Yang (1979) |
|
| Hongpei Zhang et al. (2004) |
|
| Xinhui Liu et al. (2010) +1 other reference |
|
| Hongyang Li et al. (2007) +1 other reference |
|
| Li et al. (2023) |
|
| Jianguo Yang et al. (2005) |
|
| Bingbin Yang (1979) |
|
| Guangxi Zhao (2007) |
|
| Bingbin Yang (1979) |
|
| Mao Jingwen et al. (1999) |
|
| Jianzhong Feng et al. (2002) |
|
| Hongjie Kang et al. (2008) |
|
| Jinchun Li et al. (2005) +3 other references |
|
| Hongjie Kang et al. (2008) |
|
| Donghong Zhao et al. (2004) |
|
| Mingzheng Zhuang (1983) +1 other reference |
|
| Changming Liu and Zhenyu Fan (2013) |
|
| Ruan Ting (1984) +1 other reference |
|
| Yi Li (2001) |
|
| Lin Niu (1994) |
|
| Chunlin Yang (1993) |
|
| Maozhong Min et al. (1987) |
|
| Qian Zhang et al. (2008) |
|
| Yu Chen et al. (1984) |
|
| Yonghong Zhu (2010) |
|
| Deru Xu et al. (2008) +1 other reference |
|
| Guoxi Ma (1999) |
|
| Huanchun Duan (2004) +1 other reference |
- Yingshouyingzikuang District
| Yaowu Ren and Qianwen Cao (1993) |
|
| Yingjun Li et al. (2007) |
|
| Guanxiang Song (1991) |
|
| Sun et al. (2022) |
|
| Yongwen Shi (2009) |
|
| pubs.usgs.gov (2006) |
|
| Jun Liu et al. (2009) +2 other references |
|
| Ruofeng Gao et al. (2008) |
|
| Li et al. (2018) +1 other reference |
|
| Li Liu et al. (2010) |
|
| Zhixia Pei et al. (2004) |
|
| Mingliang Xu et al. (2006) |
|
| Dingsheng Zhao (2011) |
|
| Yanjing Chen et al. (2008) |
|
| Yanjing Chen et al. (2004) |
|
| Fengjun Bai et al. (2009) |
|
| Fengjun Bai et al. (2009) |
|
| Fengjun Bai et al. (2009) |
|
| Fengjun Bai et al. (2009) |
|
| Diankai Chen and Danshen Zhou (1984) |
|
| Diankai Chen and Danshen Zhou (1984) |
|
| Shangyin Zhou et al. (2007) |
|
| Guang Wu et al. (2013) |
|
| Mineralogy Society of Hong Kong (Website) |
- Ma On Mountain (Ma On Shan)
| McQueen et al. (1995) |
|
| www.smartminerals.com (2004) |
|
| Yuanming Pan and Ping Dong (1999) |
|
| www.smartminerals.com (2004) |
|
| Luanling Liu and Junhua Ding (1987) |
- Shennongjia Forest District
| Xiangjin Meng et al. (2004) |
|
| Jurui He et al. (1992) |
|
| Qianglu Zhang and Lisheng Liu (2006) |
|
| Dongsheng Yue et al. (1994) |
|
| Qiong Cao et al. (2014) |
|
| Kamitani et al. (2007) |
|
| Dapeng Wang et al. (2007) |
|
| Xuexiang Gu et al. (2003) +1 other reference |
- Miaoershan-Yuechengling ore field
| Xinquan Xie and Henglong Zhang (2005) |
- Alxa League (Alashan Prefecture)
| Hong Liu (1986) |
|
| Daming Bai et al. (2006) |
- Baotou City (Baotou Prefecture)
- Darhan Muminggan United Banner (Da'erhan Maoming'an Lianheqi; Damaoqi)
| Chao Tang et al. (2013) |
|
| Chao Tang et al. (2013) |
- Bayannur City (Bayannao'er Prefecture)
- Urad Rear Banner (Wulate Houqi)
| Zhaolong Li et al. (1981) |
|
| Zhaolong Li et al. (1981) |
|
| Yuqi Wang et al. (2013) |
- Chifeng City (Ulanhad League; Chifeng Prefecture)
- Harqin Banner (Kalaqin Co.)
| Junping Wang (2012) |
- Hexigten Banner (Keshiketeng Co.)
| Luo et al. (2014) |
|
| Yi Xu et al. (2008) |
|
| Danyang Liu (1991) |
|
| Shuyin Niu et al. (2006) +2 other references |
- Hinggan League (Xing'an Prefecture)
| Xixin Zhang (1989) |
- Hohhot City (Huhehaote Prefecture)
| Youbin Liang (1981) |
- Hulunbuir City (Hulunbei'er Prefecture)
| Wang et al. (2024) |
- New Barag Right Banner (Xin Barag Youqi)
| Libo Hao et al. (1994) |
- Xilingol League (Xilinguole Prefecture)
- East Ujimqin Banner (Dongwuzhumuqin Co.; Dongwu Qi)
| Fengjun Nie et al. (2004) +1 other reference |
|
| Fengjun Nie et al. (2007) |
|
| Peng Hu et al. (2005) |
|
| Xiulan Nie and Wanrong Hou (2010) |
|
| Hongli Liu et al. (2011) |
- Sonid Left Banner (Sunite Zuoqi)
| Yong Zhang et al. (2013) |
|
| Shi |
- Sonid Right Banner (Sunite Youqi)
| Liangsheng Ge et al. (2012) |
- West Ujimqin Banner (Xiwuzhumuqin Co.)
| Xiaofei Pan et al. (2009) +1 other reference |
|
| pubs.usgs.gov (2006) |
|
| Xia Ai and Zengyi Chen (1993) |
|
| Yunhuai Lin (1986) |
|
| Zhongling Wang (1991) +1 other reference |
|
| Zhongling Wang (1987) |
- Dahutang W-(Sn) ore field
| Yang et al. (2022) |
|
| Bangsheng Tian and Buyun Yuan (2008) |
- Xiushui Uranium ore field
| Dahlkamp et al. (2009) |
|
| Dahlkamp et al. (2009) |
- Dexing Cu-Mo-Au ore field
| Kuanglin Yan (1978) |
|
| Chunsheng Lin and Hualong Xia (2003) |
|
| Huiwu Yang and Fenggui Liu (2004) |
|
| Junliang Li et al. (2012) |
|
| Xingyun Zhang et al. (2001) |
|
| Tian et al. (2024) |
- Jiangyuan District (Hunjiang Co.)
| Yajian Liu et al. (2008) |
|
| Lixia Kang et al. (2008) |
|
| Shuyi Chou (2011) |
|
| USGS Open-File Report 03-220 |
- Xiaogushan Pb-Zn-Au-Ag deposit
| Cunjie Dong et al. (2010) |
|
| Cunjie Dong et al. (2010) |
- Jinchanggouliang-Erdaogou ore field
| Peng Wang et al. (2013) |
|
| Dejuan Wen et al. (2013) |
|
| Guangcheng Chi and Ailin Zhao (2006) |
|
| Minzhi Yang (2003) |
|
| Guangyu Zhou (1983) |
|
| Hongyu Li et al. (2009) |
|
| Hongyu Li et al. (2009) |
- Golog Tibetan Autonomous Prefecture
| Duan Guolian (1991) |
- Gyêgu Autonomous Prefecture (Yushu Autonomous Prefecture)
| Zongxue Zheng et al. (2012) |
|
| Li Wenming et al. (2008) |
|
| Baohua Li et al. (2006) |
|
| Chenglong Zhao et al. (2013) +1 other reference |
|
| Zhiyong Liu et al. (2006) |
|
| Zhiyong Liu et al. (2006) |
|
| Zhiyong Liu et al. (2006) |
- Haibei Tibetan Autonomous Prefecture
| Bingbin Yang (1979) |
|
| Jun Liu et al. (2006) |
|
| Peng Wu et al. (2012) |
|
| Jiangfeng Liu (2009) |
- Xunhua Salar Autonomous County
| Xiangping Dong et al. (2010) |
- Haixi Mongol and Tibetan Autonomous Prefecture
- Da Qaidam (Dachaidan Co.)
| Dequan Zhang et al. (2007) |
|
| Wenguang Yao et al. (2002) +2 other references |
|
| Peilin Xue et al. (2006) |
|
| Changfu Gan et al. (2012) |
|
| Peilin Xue et al. (2006) |
|
| Xuehu Li (2010) |
- Golmud City (Ge'ermu Co.)
| Li et al. (2021) |
- Ka'erqueka Cu-polymetallic deposit
| Zhongrong Liu et al. (2010) +1 other reference |
|
| Zhuangzhi Qian et al. (2004) +1 other reference |
- Tuotuohe area (Tuotuo river area)
| Sun +6 other references |
|
| Yunhua Liu et al. (2005) |
- Mangnai City (Mangya Co.)
| Shanfa Xu et al. (2004) |
|
| Shuyue He et al. (2008) |
- Qaidam Basin (Chaidan Basin; Tsaidam Basin)
| Dequan Zhang et al. (2005) |
- Huangnan Tibetan Autonomous Prefecture
| Yalong Kang et al. (2013) |
|
| Xiaoming Fu et al. (2009) +1 other reference |
|
| Yinjie Gong et al. (2013) |
|
| Huaikui Tu (1998) |
|
| Xishe Zhang (2012) +1 other reference |
|
| Jusheng Gao and Lianhong Chen (2006) +1 other reference |
|
| Ruiting Wang et al. (2002) |
|
| Jun Zhang et al. (2008) |
|
| Licheng Tang (1989) +1 other reference |
|
| Yaxiong Zhong (1980) |
|
| Metallurgical Research Team of the Geological Bureau of Shandong (1978) |
|
| Decheng Zhu et al. (2007) |
- Muping-Rushan metallogenic belt
| Jianchao Liu et al. (2010) |
|
| Yuan Zhang et al. (2008) +2 other references |
|
| Yuan Cong et al. (2005) +1 other reference |
|
| Yaxiong Zhong (1980) |
|
| Zhaolong Li and Lianying Zhang (1999) +1 other reference |
|
| Jiannian Zeng (1991) |
|
| Zhian Qin and Keqin Xue (2003) |
|
| Zhian Qin and Keqin Xue (2003) |
|
| Caiping Chen (2006) |
|
| Jiying Li (1986) +1 other reference |
- Garzê Autonomous Prefecture (Ganzi Autonomous Prefecture)
| Huaping Zhu (2007) |
|
| Qingmin Jin et al. (1992) |
|
| Jian Liu (2009) |
|
| Jianzhong Li et al. (2006) +1 other reference |
|
| Zhimin Cao (1991) |
|
| Chenghua Zhang and Guodong Ji (1983) |
|
| Pei et al. (2024) |
|
| Xu Chen et al. (2008) |
|
| Zhibin Gao (1982) |
|
| Yongjun Wang et al. (2012) |
|
| Wen Qi et al. (2006) +1 other reference |
|
| Bangguo Zhou et al. (2013) |
|
| Guangdi Zhang et al. (2002) +2 other references |
- Lalachang Cu deposit (Lala Cu deposit)
| Zhimin Zhu et al. (2009) |
|
| Kamitani et al. (2007) |
- Quezhushan Pb-Zn ore field
| Wei Li et al. (2010) |
- Ngawa Autonomous Prefecture (Aba Autonomous Prefecture)
- Zoigê Co. (Ruo'ergai Co.)
| Su Sun (1993) |
|
| Liangsheng Ge (1997) |
|
| Yanguo Teng et al. (2000) |
|
| Ziyi Wan (1986) |
- Maizhokunggar Co. (Mozhugongka Co.)
| Lu et al. (Cu) |
|
| Tingting Liu et al. (2011) |
|
| LENG et al. (2014) +2 other references |
|
| Jin Duan and Pengfei Wu (2012) |
|
| Zhiying Huang and Guangming Li (2004) |
|
| Zengqian Hou et al. (2009) |
|
| Singer et al. (2008) |
|
| Qingsong Zhang and Weide Zhou (2006) +1 other reference |
|
| Xiaochun Wang et al. (2002) +1 other reference |
- Nagchu Prefecture (Naqu Prefecture)
| Ziyi Wan (1986) |
|
| Liu et al. (2024) |
|
| Liqiang Wang et al. (2012) |
|
| Liu et al. (2024) |
|
| Liu et al. (2024) |
|
| Shengrong Li et al. (2005) |
|
| Liu et al. (2023) |
- Nixiong Fe-Cu ore field (Nyixung Fe-Cu ore field)
| Yushuai Yu et al. (2012) |
|
| Jiang +8 other references |
|
| Biaowu Xie et al. (2012) +1 other reference |
|
| Zhenghua Hu et al. (2012) |
|
| Liu et al. (2024) |
|
| Guangming Li et al. (2006) +1 other reference |
|
| Liu et al. (2024) |
|
| Shengrong Li et al. (2005) +1 other reference |
- Nyingtri Prefecture (Linzhi Prefecture)
- Gongbo'gyamda Co. (Gongbujiangda Co.)
| Congxuan Kang et al. (2013) |
- Qamdo Prefecture (Changdu Prefecture)
| Liu et al. (2024) |
|
| pubs.usgs.gov (2006) |
- Shannan Prefecture (Lhokha Prefecture; Lhoka Prefecture)
| Jianfang Zhang et al. (2010) +2 other references |
- Nêdong Co. (Naidong Co.; Zedang Co.)
- Kelu-Chongmuda Cu-Au belt
| Guangming Li et al. (2006) |
- Kelu-Chongmuda Cu-Au belt
| Yushui Chen et al. (2011) |
- Kelu-Chongmuda Cu-Au belt
| Shaohuai Wang (2006) +1 other reference |
- Xigazê Prefecture (Rikaze Prefecture; Shigatse Prefecture)
- Namling Co. (Nanmulin Co.)
| Youye Zheng et al. (2007) |
- Ngamring Co. (Angren Co.)
| Qi et al. (2021) |
|
| Liu et al. (2024) |
|
| Singer et al. (2008) +1 other reference |
- Xaitongmoin Co. (Xietongmen Co.)
| Yushuai Yu et al. (2011) +1 other reference |
|
| Xinghai Lang et al. (2011) |
|
| Liu et al. (2024) |
- Akesu Prefecture (Aksu Prefecture; Aqsu Prefecture)
| Bingbin Yang (1979) |
|
| Bingbin Yang (1979) |
- Bayin'gholin Autonomous Prefecture (Bayingolin Autonomous Prefecture; Bayinguoleng Autonomous Prefecture)
| Wuhan Huaze Science & Technology ... |
|
| Wuhan Huaze Science & Technology ... |
|
| Zuoheng Zhang et al. (2012) |
|
| Zhiliang Wang et al. (2004) |
- Bo'ertala Autonomous Prefecture (Börtala Autonomous Prefecture)
| Zhiye Jia et al. (2011) |
- Wenquan Co. (Arixang Co.; Arishang Co.)
| Liu et al. (2022) |
|
| Liu et al. (2022) |
- Changji Autonomous Prefecture (Sanji Autonomous Prefecture)
- Qitai Co. (Gucheng Co.; Guqung Co.)
| Hengxiang Yu et al. (1998) |
- Hami Prefecture (Kumul Prefecture; Qumul Prefecture)
| Denghong Wang et al. (2009) |
|
| Jinxiang Li et al. (2007) |
|
| Chunji Xue et al. (2000) |
|
| Chen Wen et al. (2006) |
- Huangshan-Jing'erquan ore belt
| Jingwen Mao et al. (2007) |
|
| Jinzhu San et al. (2007) |
|
| Yan Sun et al. (2009) |
|
| Qingli Wang et al. (2008) |
|
| Zhengen Zhang and Li Ma (1994) |
- Tuwu-Yandong copper ore belt
| Fengjun Nie et al. (2004) |
|
| Lianchang Zhang et al. (2004) |
|
| Weixuan Fang et al. (2003) |
- Hetian Co. (Khotan Co.; Hotan Co.; Hoten Co.)
| Wen Qi et al. (2005) |
- Pishan Co. (Pixina Co.; Guma Co.)
| Pinlong Du (1984) |
- Kashi Prefecture (Kashgar Prefecture; Qeshqer Prefecture)
- Jiashi County (Peyziwat Co.; Payzawat Co.)
| Sicheng Wang et al. (2011) |
- Tashiku'ergan Co. (Taxkorgan Co.; Tashqurqan Co.)
| Jianguo Huang et al. (2009) |
- Kezilesu Autonomous Prefecture (Kizilsu Autonomous Prefecture; Qizilsu Autonomous Prefecture)
- Aketao Co. (Akto Co.; Aqto Co.)
| Jingchun Wang and Nan Fang (2005) |
|
| Wenhua Ji et al. (2009) |
- Wuqia Co. (Ulugqat Co.; Ulughchat Co.)
| Minghua Zheng et al. (2002) |
|
| Wenzhang Fu and Jinchuan Gu (2001) |
|
| Zhiliang Wang et al. (2003) |
|
| Guanghai Liu et al. (2000) +4 other references |
- Wulumuqi Prefecture (Ürümqi Prefecture; Ürümchi Prefecture)
- Wulumuqi Co. (Ürümqi Co.; Ürümchi Co.)
| Bingbin Yang (1979) |
- Yili Hasake Autonomous Prefecture (Ili Kazakh Autonomous Prefecture)
- Aletai Prefecture (Altay Prefecture)
| Chunming Han et al. (2007) |
|
| Fuquan Yang et al. (2008) |
|
| Taide Li (2002) +1 other reference |
- Qinghe Co. (Qinggil Co.; Chinggil Co.)
| Fuquan Yang et al. (2010) |
|
| Shujun Lü et al. (2012) |
|
| Huiliang Xiao et al. (2002) |
|
| Zhanfeng Zhao et al. (2009) +1 other reference |
- Nileke Co. (Nilka Co.; Nilqa Co.)
| Chen et al. (2007) |
|
| He Zhang et al. (2012) |
|
| Zongliu Lu and Jiangping Mo (2006) |
|
| Chunlong Wang et al. (2012) |
|
| Chuanmin Sun et al. (2002) +1 other reference |
|
| Yongbei Zhang et al. (2003) |
|
| Yongbei Zhang et al. (2003) |
|
| Xiao'ou Zhao (1989) +1 other reference |
- Machangqing Cu-Mo-Au ore field (Baoxingchang Cu-Mo-Au ore field)
| Liangsheng Ge et al. (2002) +1 other reference |
|
| Liangsheng Ge et al. (2002) +1 other reference |
|
| Yong Wang et al. (2006) |
|
| Yong Wang et al. (2006) |
- Dêqên Autonomous Prefecture
- Dêqên County (Deqin Co.; Diqing Co.)
| Xian Yang et al. (2013) |
|
| Siyao Chen et al. (2013) +2 other references |
|
| Wenchang Li et al. (2012) |
|
| He et al. (2022) |
|
| Baogui Zhang et al. (2002) |
|
| Jun Gao and Yinliang Cui (2004) +1 other reference |
|
| Zicheng Wu et al. (2013) |
|
| Mu Yang and Shenglin Peng (2006) |
|
| Hercule Shen |
|
| Hercule Shen |
|
| Chen (2002) |
|
| Mu Yang and Shenglin Peng (2006) |
|
| Bugao Xue (2007) |
|
| Mu Yang and Shenglin Peng (2006) +1 other reference |
|
| Deli Liu et al. (2008) +1 other reference |
|
| Guangjun Li et al. (2013) |
|
| Longqing He et al. (2009) |
|
| Jiajun Liu et al. (2010) +1 other reference |
|
| Jiajun Liu et al. (2010) |
|
| Liu Jiajun et al. (2001) |
|
| 高翔 et al. (2013) |
|
| Wen Yan and Chaoyang Li (1992) |
|
| Wen Yan and Chaoyang Li (1992) |
|
| Wen Yan and Chaoyang Li (1992) |
|
| Bugao Xue (1996) +1 other reference |
|
| Rongjun Si et al. (2013) |
|
| Mu Yang and Shenglin Peng (2006) |
|
| Mu Yang and Shenglin Peng (2006) |
|
| Mu Yang and Shenglin Peng (2006) |
- Yimen Cu ore field (Sanjiachang Cu ore field)
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Bugao Xue (1996) |
|
| Qian Zhang et al. (2008) |
|
| Qian Zhang et al. (2008) |
|
| Chen (2002) |
|
| Qian Zhang et al. (2008) |
|
| Huijin Lu and Jian Wang (2005) |
|
| Huijin Lu and Jian Wang (2005) |
|
| Lina Peng et al. (2009) |
|
Colombia | |
|
| Altenberger et al. (2022) |
- Norte de Santander Department
| Publications of the Field Columbian ... |
|
Croatia | |
|
| Čepelak |
|
| Bakarna rudna pojava kod Kraljičinog ... |
|
| Palinkaš +4 other references |
|
Cuba | |
- Santiago de Cuba Province
| Rocks & Min.: 20:169. |
|
Cyprus | |
|
| Dmitry Tonkacheev collection |
|
Czech Republic | |
|
| Sejkora et al. (2021) |
|
| Velebil (Dě) +4 other references |
|
| Lapis 2000 (4) |
|
| Pauliš P. Mineralogické lokality ... |
|
| Litochleb +4 other references |
|
| • Velebil |
|
| Velebil D et al. (5) |
|
| P. Škácha |
|
| [Lapis 1995 |
|
| Bradna +4 other references |
|
| Duda |
- Rychnov nad Kněžnou District
- Rokytnice v Orlických horách
| Velebil |
|
| ... |
|
| |
|
| Beran (Ag, Au, Co) +2 other references |
|
| Sejkora et al. (2006) |
- Jablonec nad Nisou District
| Rare Cu minerals |
- Rychnov u Jablonce nad Nisou
| Syka |
|
| Fengl +3 other references |
|
| Žáček |
|
| Kruťa |
|
| Fojt (A) |
|
| Novák |
|
| Sejkora (1994) |
|
| Kruťa |
|
| Fojt (A) |
|
| Kruta |
|
| Kuttna +1 other reference |
|
| Losos et al. (Silesicum, Czech Republic) |
|
| Dvořák |
|
| Losert |
|
| Fengl (2.) |
|
| Fiala V. (1977) |
|
| Pauliš P. Nejzajímavější ... |
|
| Pauliš +3 other references |
|
| Láznička P.: Mineralogické výskyty ... |
|
| Láznička P.: Mineralogické výskyty ... |
|
| Pačes T (1958) |
|
| Malý (svratecká klenba moravika) |
|
| Malý (svratecká klenba moravika) |
|
| Malý |
|
| Houzar |
|
| • Dolníček |
|
| Slavíček |
|
| none |
|
| mineral.-petrolog. Odd. Nár. Muz. (Praha) +2 other references |
|
| Sejkora (1998) |
|
| Sejkora (1994) |
|
| Malý (svratecká klenba moravika) |
|
| Houzar |
|
| Houzar S. |
- Žďár nad Sázavou District
| |
- Uherské Hradiště District
| Pauliš |
|
Dominican Republic | |
|
| Colomer et al. (2013) |
|
| Copper Handbook 1911 |
|
DR Congo | |
|
| Lapis 17 (3) |
|
| Anybody Collection |
|
| Kampunzu et al. (2009) |
|
| Deliens (1996) |
|
| Saleem et al. (2016) |
|
| Deliens (1996) +1 other reference |
|
| Mambwe et al. (Ruashi Cu-Co Deposit) |
|
| Kampunzu et al. (2009) |
|
| El Desouky et al. (2008) |
|
| Xiangqian Li et al. (2010) +1 other reference |
|
| Paul De Bondt collection |
|
| Wilson (2018) |
|
| Deliens (1989) |
|
| Pascal Mambwe et al. (2018) |
|
Ecuador | |
|
| Singer et al. (2008) |
|
| Collected by Alejandro Félix |
|
Egypt | |
|
| A. El-Taher et al. (2003) |
|
| Cox et al. (2003) |
|
| Faisal et al. (2021) |
|
| Faisal et al. (2021) |
|
| Richard de Nul collection |
|
| Helmy et al. (1995) |
|
| Cox et al. (2003) |
|
| Afify et al. (2022) |
|
| Afify et al. (2022) |
|
| Cox et al. (2003) |
|
Eswatini | |
|
| Cairncross (2004) |
|
Europe | |
|
| Jansa et al. (Č) +4 other references |
|
Federated States of Micronesia | |
|
| Dr. Kameki Kinoshita collection (curated at Geological Survey of Japan) |
|
Finland | |
|
| Ilkka Mikkola collection |
|
| Joel Dyer Photo ID: 710887 |
- Kevitsansarvi (Keivitsansarvi)
| Mutanen (1997) |
|
| Sandström et al. (2009) |
|
| Raman spectroscopy by Joel Dyer 2019 |
|
France | |
|
| Gol D. (2009) |
|
| Belot (1978) |
|
| Gol (2010) |
|
| Belot (1978) |
|
| C. Vialaron : La mine d'antimoine de Daü et al. (1999) |
|
| Pélisson (1989) |
|
| erik vercammen field observations and ... |
|
| Henwood (1871) |
|
| Rouveyrol P. (1961) |
|
| Mickael Landrez |
|
| J. Geffroy |
|
| Duarte (2014) |
|
| Designolle J-L. (1993) |
|
| Personnaly collected by M. Diot in 2009 |
|
| Lacroix (1893-1913) +1 other reference |
|
| Collected by Michel Blondieau |
|
| BRGM (1980) |
|
| BRGM (1981) |
|
| BRGM (1981) |
|
| BRGM (1981) |
|
| Jorelle (2002) |
|
| BRGM (1981) |
|
| Belot (1978) |
|
| G. Aubert : "Les coupoles granitiques de Montebras et d'Echassières (Massif Central Français) |
|
| J. Chervet |
|
| Chollet Pascal Collection |
|
| Palache et al. (1951) +2 other references |
|
| Chollet Pascal Collection |
|
| Gastineau (1999) |
|
| Favreau G. et al. (1996) |
|
| Fiche BSS 06493X4002/GT |
|
| ficheinfoterre.brgm.fr (2021) |
|
| Guichon (2017) |
|
| De Ascençao Guedes et al. (2000) |
|
| P Le Roch self collected |
|
| De Ascençao Guedes et al. (2000) |
|
| De Ascençao Guedes et al. (2000) |
|
| self collected by Pierre Le Roch. |
|
| De Ascençao Guedes et al. (2012) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Le regne mineral - Hors série N° XIII ... +1 other reference |
|
| yves Mourey |
|
| Collection Yves Mourey |
|
| Yves Mourey Collection |
|
| MOUREY Y. (2017) |
|
| OLLIC Pascal Collection |
|
| Chollet Pascal Collection |
|
| |
|
| |
|
| ASSELBORN E. (1982) |
|
| Collective (2007) |
|
| Kolitsch (1997) |
|
| Mikael Gonzales |
|
| Chauris (2014) |
|
| Pierrot et al. (1973) +1 other reference |
|
| Fiche BSS BRGM n° 11183X4002/GT. +1 other reference |
|
| Wittern et al. (Cologne) |
|
| A. Wittern et al. (Cologne) +1 other reference |
|
| A. Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) +1 other reference |
|
| Wittern et al. (Cologne) +1 other reference |
|
| Nicolas Meisser |
|
| Wittern et al. (Cologne) |
|
| Paul DeBondt and Thierry Brunsperger ... |
|
| T. Brunsperger In-Situ observations. |
|
| T. Brunsperger collection. |
|
| T. Brunsperger observations |
|
| Wittern |
|
| Wittern |
|
| Wittern |
|
| Th Brunsperger collection |
|
| Wittern |
|
| Hohh (1994) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Hohl: "Minéraux et Mines du Massif Vosgien" (Mulhouse) |
|
| Belot (1978) +1 other reference |
|
| Alain Schaefer collection |
|
| Th. Brunsperger Collection |
|
| Mines |
|
| Wittern et al. (Cologne) |
|
| Hohh (1994) |
|
| Hohh (1994) |
|
| Hohh (1994) |
|
| Hohh (1994) |
- Longeville-lès-Saint-Avold
| |
|
| ESCANDE et al. (1973) +6 other references |
|
| ESCANDE et al. (1973) +6 other references |
|
| J.-L. Hohl "Minéraux et Mines du Massif Vosgien" (Mulhouse) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
|
| Wittern et al. (Cologne) |
- District de La-Croix-aux-Mines
| Wittern et al. (Cologne) |
|
| Briggs (1975) |
|
| Briggs (1975) |
|
| Briggs (1975) |
|
| Briggs (1975) |
|
| Briggs (1975) |
|
| Briggs (1975) +1 other reference |
|
| Briggs (1975) |
|
| |
|
| Thiery et al. (2020) |
|
| Belot (1978) |
|
| Remy Ph. (2003) |
|
| Inventaire Mineralogique de la France ... |
|
| Self collected Jean-Marie LAURENT - ... |
|
| Inventaire mineralogique de l'Ariege ( Edition BRGM 1984) |
|
| Gol (2014) |
|
| Gol (1998) |
|
| Berbain et al. (2000) |
|
| Forner et al. (1997) |
|
| Berbain C. & Aymar J. (1991) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Les Anciennes mines de Padern - Montgaillard ( Aude ) |
|
| BERBAIN Ch. et al. (2006) |
|
| Belot (1978) |
|
| Berbain et al. (2005) |
|
| www.zampano.com (n.d.) +1 other reference |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| BSS BRGM 09615X4005/GT |
|
| collection BONNET Frederic |
|
| Gol (2015) |
|
| Queneau (n.d.) |
|
| Georges FAVREAU collection + EDX ... +1 other reference |
|
| Lheur (2023) |
|
| Leconte Jean +1 other reference |
|
| Leconte J. et al. (2016) |
|
| Jean-Marie LAURENT collection |
|
| Favreau et al. (2010) |
|
| Queneau (n.d.) |
|
| Chollet Pascal collection - XRD ... |
|
| Inventaire Minéralogique de la France ... |
|
| Jean Claude Dol |
|
| Le Cahier des micromonteurs |
|
| Queneau (n.d.) |
|
| Chollet Pascal Collection |
|
| Gol et al. (2010) |
|
| Gol et al. (2010) |
|
| Gol et al. (2010) |
|
| Le Règne Minéral 47: 5-21 |
|
| Robin Fialip 2013 |
|
| Lacroix Minéralogie de la France |
|
| carte geologique 50000 eme BRGM |
|
| Queneau (n.d.) |
|
| |
|
| Der Aufschluss (1974) +1 other reference |
|
| |
|
| Caubel (1997) |
|
| Gol (2010) |
|
| [Le Cahier des Micromonteurs |
|
| |
|
| collection bernard bouissac/antoine ... |
|
| Self collected by S. MAURY |
|
| Aufschluss 1974 (3) |
|
| Boisson et al. (2014) |
|
| Rateau (2009) |
|
| H.GERMAIN (1983) |
|
| GRITTI C. (1970) |
|
| Queneau (n.d.) |
|
| M.BERNATSKY (1974) |
|
| BERBAIN et al. (2010) +1 other reference |
- Ravin de la font de Paris
| Berbain +2 other references |
|
| Laumonier (2015) |
|
| Berbain et al. (2005) |
|
| Le Cahier des Micromonteurs |
|
| erbain +2 other references |
|
| Dubru. M (1986) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Guitard (2010) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Berbain et al. (2005) |
|
| Self collected J.M Laurent - 2005 |
|
| Laurent J-M. (2003) |
|
| Jean-Marie LAURENT collection |
|
| PIERROT et al. (1976) |
|
| PIERROT R. et al. (1976) |
|
| desescaut.jy@voila.fr |
|
| desescaut.jy@voila.fr |
|
| Baret (1905) |
|
| Baret (1905) |
|
| Leydet (1998) |
- Provence-Alpes-Côte d'Azur
| De Ascenção Guedes (2011) +1 other reference |
|
| Chollet Pascal Collection |
|
| "Les minéralisations associées au volcanisme andésitique de Biot-Villeneuve Loubet (Alpes Maritimes) |
|
| Belot (1978) |
|
| Pierrot et al. (1974) +1 other reference |
|
| Pierrot et al. (1974) +1 other reference |
|
| R. Pierrot |
|
| R. Pierrot |
|
| Mari et al. (1997) |
|
| Belot (1978) |
|
| Legrand et al. (1986) +1 other reference |
|
| R. Pierrot |
|
| Belot (1978) |
|
| Maury (2010) +1 other reference |
|
| Pierrot et al. (1974) +1 other reference |
|
| Pierrot et al. (1974) +1 other reference |
|
| Belot (1978) |
|
| R. Pierrot |
|
| Belot (1978) |
|
| R. Pierrot |
|
| Pierrot et al. (1974) +1 other reference |
|
| Pierrot et al. (1974) +1 other reference |
|
| Pusztaszeri (1969) |
|
| Pierrot et al. (1972) +1 other reference |
|
| R. Pierrot |
|
| Belot (1978) |
|
| |
|
| Self collected S. MAURY 2013 |
|
| Pierrot et al. (1972) +1 other reference |
|
| R. Pierrot |
|
| Pierrot et al. (1972) +1 other reference |
|
| R. Pierrot |
|
| Self collected S. Maury |
|
| Belot (1978) |
|
| Pierrot et al. (1972) |
|
| Belot (1978) |
- Saint-Maurice-en-Valgodemard
| R. Pierrot |
|
| R. Pierrot |
- Saint Daumas - Pic Martin mines
| Mari et al. (1979) |
|
| Mari et al. (1979) |
|
| Mari et al. (1979) |
|
| Mari et al. (1979) |
|
| Mari et al. (1979) |
|
| Self collected by S. MAURY +1 other reference |
|
| Mari et al. (1979) |
|
| Self collected by S. MAURY |
|
| Jean Marc Cottet collection |
|
| self collected S. MAURY 2008-2009 |
|
| Mari et al. (1979) |
|
| Jean-Marie Claude collection +3 other references |
|
| Jean-Marie Claude collection. |
|
| Stephane Maury Collection |
|
| ie Note de Mr. F. Pisani (1860) |
|
Germany | |
- Badenweiler Pb mining district
| Weiß (1990) |
|
| Emser Hefte 15 (3) |
|
| Markl (2017) |
|
| Steen (2005) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Gruber (2000) |
|
| Schlomann et al. (1990) |
|
| H. Steen: Erzgräber 11 (1997) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Lapis 21 (12) |
|
| |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| geni 2010 |
|
| Aufschluss 71 (3) |
|
| Wittern (1995) |
|
| [Wittern (1995) |
|
| Wittern (2001) |
|
| Lapis 1984 (1) |
|
| Walenta (1992) |
|
| Schlomann et al. (1991) |
|
| Wittern (2001) |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| Lapis 21 (12) +1 other reference |
|
| |
|
| |
|
| Neschen (n.d.) |
|
| |
|
| Walenta (1992) |
|
| Hofmann (1979) |
|
| Meinrad Kempf Collection |
|
| Walenta (1992) |
|
| Weiß (1990) |
|
| 54. +1 other reference |
|
| American Mineralogist: 70: 214 +1 other reference |
|
| Neschen (n.d.) |
|
| www.frischglueck.de (2002) |
|
| www.mineralienatlas.de (n.d.) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| |
|
| Wittern (2001) |
|
| Wittern (2001) |
- Rollenberg railway tunnel
| Weiß (1990) |
|
| Lapis (2) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Nickel et al. (1985) |
|
| Weiß (1990) |
|
| Lapis 8 (4) |
|
| Obermüller et al. (1993) |
|
| ... |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| ... |
|
| see locality page |
|
| 70. +2 other references |
|
| |
|
| Lorenz et al. (2007) |
|
| Hock et al. (1992) |
|
| Weiß (1990) |
|
| Meier (1995) |
|
| Weiß (1990) |
|
| 90. +1 other reference |
|
| Klaus Ludwig Collection |
|
| Kalus Ludwig |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Dill et al. (2010) |
|
| Nickel et al. (1985) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| In the collection of Brent Thorne. ... |
|
| Collection of Steffen Michalski |
|
| Collection of Steffen Michalski |
|
| Thorne (n.d.) |
|
| Collection of Steffen Michalski |
|
| Collection of Steffen Michalski |
|
| Collection of Steffen Michalski |
|
| Lapis 25 (2) |
|
| Collection of Steffen Michalski |
|
| Belendorff et al. (1987) |
|
| Collection of Steffen Michalski |
|
| Collection of Steffen Michalski |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Nickel et al. (1985) |
|
| Blessing et al. (1991) |
|
| Weiß (1990) |
|
| |
|
| Emser Hefte 1988 (1) +1 other reference |
|
| www.mineralienatlas.de (n.d.) |
|
| Nickel et al. (1985) |
|
| Emser Hefte 1982 (2) |
- Dillenburg mining district
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Legner et al. (2022) |
|
| Weiß (1990) |
|
| Vanrusselt (2023) |
|
| P Haas collection |
|
| Blaß et al. (1993) |
|
| Andreas Gerstenberg |
|
| Weiß (1990) |
|
| Schnorrer (1995) +2 other references |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| Wittern (2001) |
|
| Erik Hock Collection +1 other reference |
|
| Wittern (2001) |
|
| www.mineralienatlas.de (n.d.) |
|
| Weiß (1990) |
|
| Schnorrer et al. (2009) |
|
| J. Gröbner: Neufunde aus den Bergbaurevieren St. Andreasberg et al. (2007) |
|
| Weiß (1990) |
|
| Wittern et al. (1986) +1 other reference |
|
| conrad Blömeke: Über die ... |
|
| Weiß (1990) |
|
| |
|
| Gröbner et al. (2011) |
|
| |
|
| Gröbner et al. (2011) |
|
| C.Auer (2021) |
|
| Erik Vercammen collection |
|
| |
|
| Schnorrer et al. (2002) +1 other reference |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| J. Gröbner (2001) |
|
| Andreas Gerstenberg collection |
- Harz (Landkreis Göttingen)
| |
|
| Weiß (1990) |
|
| Hotze (1989) +1 other reference |
- Hagen am Teutoburger Wald
| Blaß et al. (2021) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| D. Pawlowski: "Mineralfundstellen im Sauerland" (Munich) |
|
| Weiß (1990) |
|
| Neschen (n.d.) |
|
| Weiß (1990) |
|
| www.mineralienatlas.de (n.d.) |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| www.te-minerals.com (2008) |
|
| Lapis (2) |
|
| Weiß (1990) |
|
| www.alterbergbau.de |
|
| Wittern (2001) |
|
| Neschen (n.d.) |
|
| Weiß (1990) |
|
| |
|
| Wittern (2001) |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| D. Pawlowski: "Mineralfundstellen im Sauerland" (Munich) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Graf et al. (1982) |
|
| Graf et al. (1992) |
|
| Vetter et al. (1993) |
|
| R. Lang pers. finds |
|
| Graf et al. (1992) |
|
| Graf et al. (1982) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| W. Meyer et al. (Berlin, Stuttgart) |
- Rheinisch-Bergischer Kreis
| Weiß (1990) |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| Lapis 1988 (1) |
|
| Aufschluss 1988 (2) |
|
| Schnorrer-Köhler (1988) |
|
| Lapis 1988 (1) |
|
| M Kampf collection |
|
| Lapis 1988 (1) |
|
| Lapis 1988 (1) |
|
| Schnorrer-Köhler (1988) |
|
| Harjo Neutkens collection |
|
| M Kampf collection |
|
| Wittern (2001) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| collection Leon Hupperichs |
|
| collection Leon Hupperichs +2 other references |
|
| Liebering et al. (1883) |
|
| Volker Reppke Collection |
- Altenkirchen-Flammersfeld
| Weiss: "Mineralienfundstellen et al. (Munich) |
|
| Weiß (1990) |
|
| Wittern (2001) |
- Malscheid (Mahlscheid; Mahlscheiderkopf; Mahlscheider Kopf)
| Emser Hefte 1990 (1) |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| Emser Hefte 1987 (4) |
|
| Weiß (1990) |
|
| Arndt et al. (1920) |
|
| N. Jb. Miner. Abh. 115 (1971) |
|
| www.mgas.de (2003) |
|
| www.mineralienfreunde-der-pfalz.de (2015) |
|
| Christof Schäfer |
|
| Rahm (2014) |
|
| www.mineralienfreunde-der-pfalz.de (n.d.) |
|
| Aufschluss 62 (1) |
|
| Scheiderhöhn & Kautzsch 1936 |
|
| Mineralienverein Freisen |
|
| Lapis (2) |
|
| 50. +2 other references |
|
| Weiß (1990) |
|
| Wittern (2001) |
|
| von Beroldingen et al. (1788) |
|
| Arndt et al.: Geognostische Jahreshefte XXXI/XXXII (1920) |
|
| Lapis 2001 (6) |
|
| Hartmut Hensel Collection |
|
| Dreyer (1975) |
|
| Hartmut Hensel Collection |
|
| Lapis (10) |
|
| von Beroldingen (1788) |
|
| U. H. J. Heidtke |
|
| Lapis (5) |
|
| Lapis (5) |
|
| Wittern (2001) |
|
| Lapis (5) |
|
| Wittern (2001) |
|
| mb-minerals.de |
|
| 56. +1 other reference |
|
| Liebering et al. (1883) |
|
| Hentschel (1983) |
|
| Blass +1 other reference |
|
| Blass +1 other reference |
|
| Blass +1 other reference |
|
| Blass +1 other reference |
|
| Blass et al. (2010) |
|
| Lapis (5) |
|
| Graf et al. (1991) |
|
| Thomas Kleser Collection |
|
| Markl et al. (1990) |
|
| Lapis (9) |
|
| Lapis (3) |
|
| Weiss: "Mineralienfundstellen et al. (Munich) |
|
| Habel (2006) +1 other reference |
|
| W. Meyer et al. (Berlin, Stuttgart) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| Der Aufschluss Vol.55 |
|
| Weiß (1990) |
|
| Eberhard Riech collection |
|
| Weiß (1990) |
|
| Gerhard Müller: Bergbau-PSL-Mineralogie et al. (Saarbrücken 1996) |
|
| Thomas Kleser Collection |
|
| Thomas Kleser Collection |
|
| Thomas Kleser Collection |
|
| Weiß (1990) |
|
| Weiß (1990) |
|
| G. Müller |
|
| J. Greiner collection |
|
| |
|
| Wittern (2001) |
|
| Wittern (2001) |
|
| |
|
| Wittern (2001) |
|
| Wittern (2001) |
|
| Lapis 15 (7/8) |
|
| Thomas Lühr Collection |
|
| Gröbner et al. (2011) |
|
| Gröbner et al. (2011) |
|
| Mineralien Welt 15 Jg.Heft 2 ... |
|
| Mineralien Welt 15 Jg Heft 2-2004and 19 ... |
|
| Wittern (2001) +1 other reference |
|
| Wittern (2001) |
|
| Lapis 17 (12) |
|
| Lange |
|
| Frenzel (1874) |
|
| Massanek et al. (2005) |
|
| Müller et al. (2021) |
|
| Lapis (4) |
|
| Lapis (10) |
|
| Wittern (2001) |
|
| Tröger (2009) |
|
| Wittern (2001) |
|
| Wittern (2001) |
- Gottes Geschick Vereinigt Feld Mine
| www.dergraul.de (2001) |
|
| www.dergraul.de (2001) |
|
| Massanek et al. (2005) |
|
| Wittern (2001) |
|
| Martin et al. (2001) |
|
| Wittern (2001) |
|
| Witzke et al. (2006) |
|
| Witzke et al. (2013) |
|
| Wittern (2001) |
|
| Gröbner J. et al. (2006) |
|
| Frenzel (1874) |
|
| 90. +1 other reference |
|
| Frenzel (1874) |
|
| Wittern (2001) |
|
| Vollstädt (1979) |
|
| Wittern (2001) |
|
| A. Frenzel (1874) |
- Sächsische Schweiz-Osterzgebirge
| Mineralien-Welt (2) |
|
| Wittern (2001) |
|
| Andreas Gerstenberg collection |
|
| WEISS et al. (2001) |
|
| Lapis (7/8) |
|
| Tröger (2012) |
|
| Leithner (2008) |
|
| Andreas Gerstenberg collection |
|
| www.mineralienatlas.de (2016) |
|
| www.mineralienatlas.de (n.d.) |
|
| Weiß (1990) |
|
| Luetcke (n.d.) |
|
| T. Witzke & F. Rüger: Lapis 1998 (7/8) |
|
| Juergen Roth´s Collection +2 other references |
|
| 54. +1 other reference |
|
| Knoll (2009) |
|
| Knoll (2009) |
|
| www.kyffnet.de (n.d.) |
|
| www.kyffnet.de (2004) |
|
| Collection HJ Haas |
|
| J. Gröbner & J. Wesiger (2008) |
|
| Gröbner et al. (2008) |
|
| Wittern (2001) |
|
| T. Witzke et al.: Lapis 2001 (12) |
|
| Philip Bluemner Collection |
|
| www.mineralatlas.eu (n.d.) |
- Saalfeld-Rudolstadt District
| Rüger et al. (1992) |
|
| Leon Hupperichs collection |
|
| C. Linde |
|
| Marcus Vau Collection |
- Schmalkalden-Meiningen District
| |
|
| |
|
| |
|
| |
|
| |
|
Greece | |
|
| Frenzel (1895) |
|
| NIEDERMAYR (1982) |
- Kamariza Mines (Kamareza Mines)
| Rieck et al. (1999) +1 other reference |
|
| Chukanov et al. (2007) |
|
| |
|
| LAPIS 24 (7/8) +1 other reference |
|
| Heymann (1982) |
|
| 62 +2 other references |
|
| Geology of Ore Deposits et al. (AsO4) |
|
| Lapis et al. (1999) |
|
| Branko Rieck collection |
|
| Blaß et al. (1998) |
|
| |
|
| Matteo Chinellato collection |
|
| |
|
| |
|
| Lapis et al. (1999) |
|
| |
|
| 62 +2 other references |
|
| Lavrion Mineral Museum collection |
|
| Lavrion Mineral Museum collection |
|
| Branko Rieck collection |
|
| Branko Rieck collection |
|
| Blaß et al. (1998) |
|
| Lapis et al. (1999) |
- Kassandra mining district
| Econ Geol (1991) |
|
| Stergiou et al. (2016) |
|
| Seemann et al. (2008) |
- Eastern Macedonia and Thrace
| Christian Bracke Collection |
|
| Christian Bracke Collection |
|
| Φωτιάδης (2010) |
|
| Eliopoulos +3 other references |
|
| Robert Marschik et al. (2010) |
|
| Robert Marschik et al. (2010) |
|
| Dill et al. (2010) |
|
| Wagner et al. (1980) |
|
Greenland | |
|
| Harry et al. (1964) |
|
| Petersen (2001) |
|
| Anthony et al. (1990) |
|
| Bøggild (1953) |
|
Hungary | |
|
| Szakáll et al. (1996) |
|
| |
|
| Szakáll-Gatter-Szendrei: Mineral ... |
- Borsod-Abaúj-Zemplén County
| Sándor et al. (1997) |
|
| |
|
| Szakáll et al. (1996) |
|
| Szakáll: Minerals of Rudabánya |
|
| T Ungvári collection |
|
| Szakáll: Minerals of Rudabánya |
|
| Szakáll: Minerals of Rudabánya |
|
| T Ungvári collection |
|
| |
|
| Szakáll-Gatter-Szendrei: Mineral ... |
|
| Own found |
|
| |
|
| Szakáll-Gatter-Szendrei: Mineral ... +1 other reference |
|
| Szakáll et al. (1996) |
|
| Szakáll-Jánosi: Minerals of Hungary |
|
| AMP |
|
| Szakáll-Gatter:Hungarian Mineral ... |
|
| Sánoor Szakáll et al. (1997) |
|
| Own found 2008 +1 other reference |
|
| Szakáll-Gatter-Jánosi: Minerals of ... |
|
| GEODA 2013/2 |
|
| Szakáll et al. (1996) |
|
| Mineral Species of Hungary +1 other reference |
|
| Szakáll et al. (1996) |
|
| Szakáll et al. (1996) |
|
| Szakáll-Gatter-Szendrei: Mineral ... |
|
| Szakáll & Jánosi: Minerals of Hungary |
|
| Szakáll-Gatter-Szendrei: Mineral ... |
|
India | |
|
| Singh et al. (2002) |
|
| Singer et al. (2008) |
|
| G. K. Kesar (2011) |
- Sundargarh District (Sundergarh District)
| www.orissaminerals.gov.in (2011) |
|
| Singh et al. (1980) +1 other reference |
|
| Ahmed et al. (2018) |
|
| Ahmed et al. (2018) |
|
| Anand et al. (2022) |
|
| Ramasamy et al. (2013) |
- Palwancha (Palvancha; Paloncha)
| K. Kameswara Rao and C. Mahadevan (1960) |
|
Indonesia | |
- Central Sulawesi Province
| Singer et al. (2008) |
|
| Božidar Zalokar (1990) |
|
| Abidin (2010) |
|
| Ryann O. Collection |
- West Nusa Tenggara Province
| Kant et al. (2015) |
|
| Abidin et al. (2014) |
|
Iran | |
|
| Soughi et al. (2023) |
|
| Sadati et al. (2016) |
|
| Singer et al. (2009) |
|
| Rajabzadeh (2007) |
|
| Moradiani et al. (2024) |
|
| Bariand |
|
| Javad Amiri collection |
|
| Bariand et al. (1963) |
|
| Bariand et al. (1993) |
|
| Cherepovsky et al. (Chah Kharbuzeh-Pateyar, Torkemani localities) |
|
| mrdata.usgs.gov (n.d.) |
|
| Econ Geol (1992) |
|
| Altenberger et al. (Ag-Au) |
|
| Singer et al. (2008) |
|
| Derakhshani et al. (2009) |
|
| K. J. Henley (1970) +2 other references |
|
| Tajeddin et al. (2018) |
|
| Mirza et al. (2018) |
|
| Aliyari et al. (2009) |
|
| Zarasvandi +4 other references |
|
| Nejadhadad +3 other references |
|
| H. Zamanian et al. (2017) |
|
| Farid Mohammadi field trip |
|
| Fazli et al. (2022) |
|
| Nezafati et al. (2005) |
|
| Nezafati et al. (2005) |
|
| Nezafatis (2006) |
|
| Maghfouri et al. (2016) |
- Abbas Abad mining district
| an example of Manto type copper ... +1 other reference |
|
| Sheibi (2014) +1 other reference |
|
| Shoale et al. (2007) |
|
| Shirazi et al. (2022) |
|
| Pourkaseb et al. (2017) |
|
| Vachik Hairapetian collection +1 other reference |
|
| Shirazi et al. (2022) |
|
| Shirazi et al. (2022) |
|
| Shirazi et al. (2022) |
|
| Shirazi et al. (2022) |
|
| AN EXAMPLE OF MANTO TYPE COPPER ... +1 other reference |
|
| Shojaeddin Niroomand et al. (2011) |
|
| www.fos.ut.ac.ir (2004) +1 other reference |
|
| Singer et al. (2008) |
|
| BAZARGANI-GUILANI et al. (2008) +1 other reference |
|
| Jiba et al. (2021) |
|
| Mohsen Mohammadi |
|
| Mousavi Motlagh et al. (2024) |
|
| Maghfouri et al. (2017) |
|
Iraq | |
|
| Yassin et al. (Eds.) |
|
| Yassin et al. (Eds.) |
|
Ireland | |
|
| Min.Rec.: 20 (5) +3 other references |
|
| Henwood (1871) |
|
| Henwood (1871) |
|
| Henwood (1871) |
|
| Natural History Museum specimens |
|
| Barry Flannery (personal observations) |
|
| |
|
| Barry Flannery Collection |
|
| Cornwall |
|
| |
|
| Moreton et al. (2006) |
|
| Dr. Richard Unitt & Barry Flannery |
|
| and of serpierite at Ross Island mine +1 other reference |
|
| Barry Flannery Collection |
|
| Bergakademie Freiberg |
|
| [Mineralogical Record 30:102] |
|
| Stephen Moreton (Pers. Comm.) |
|
| Mineralogical Magazine 1959 32 : ... |
|
| Mineralogical Magazine 1959 32 : ... |
|
Israel | |
- Southern District (HaDarom District)
| Shugar et al. (eds.) |
|
| whc.unesco.org (n.d.) |
|
Italy | |
|
| AA. VV. |
|
| Del Caldo et al. (1973) |
|
| AA. VV. |
|
| Franco Luca Bonino's collection |
|
| Erik Vercammen field observation and ... |
|
| Paolo Castello: Lo Ialofane e gli altri minerali del giacimento piombo-baritico del Trou des Romains (Courmayeur, Valle d'Aosta) +1 other reference |
|
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| • Barresi et al. (2005) |
|
| De Lorenzo (1907) +1 other reference |
|
| De Michele (1974) |
|
| Larocca et al. (ed.) +1 other reference |
- Metropolitan City of Reggio Calabria
| Panichi (1911) |
|
| De Stefani (1883) |
|
| Balassone et al. (2019) |
|
| Scacchi (1879) +8 other references |
|
| Scacchi (1879) +1 other reference |
|
| Scacchi (1879) +5 other references |
|
| Scacchi (1879) +9 other references |
- Metropolitan City of Bologna
| Pedroni et al. (1996) |
|
| Pedroni et al. (1996) |
|
| Pedroni et al. (1996) |
|
| Bertolani (1953) +1 other reference |
|
| Brigatti et al. (2014) |
|
| Bartoli et al. (2008) |
|
| Bartoli et al. (2008) |
|
| Scacchetti et al. (2023) |
|
| Bertacchini et al. (1999) |
|
| ADORNI F. (1997) |
|
| Baldizzone (2008) |
|
| Giovanni Scapin Collection +1 other reference |
|
| Malagoli M. (1884) +1 other reference |
|
| Scacchetti et al. (2015) |
|
| Bortolozzi et al. (2017) |
|
| Di Colbertaldo Dino and Feruglio Giambattista (1964) +1 other reference |
|
| Feruglio G. (1966) |
|
| Zucchini (1998) |
|
| Bortolozzi et al. (2015) |
|
| Bortolozzi (n.d.) |
|
| Zucchini (1998) |
- Metropolitan City of Rome Capital
| Dario Di Domenico photo |
|
| I minerali del Museo Archeominerario "Klitsche de la Grange"Allumiere (2000) |
|
| i minerali del museo archeominerario"Klitsche de la Grange"Allumiere et al. (2000) |
|
| Di Domenico et al. (2000) |
|
| Antofilli |
|
| Ciuffardi et al. (2008) |
|
| Issel (1892) |
|
| Giovanni Scapin Collection |
|
| analysed by Dr. Anthony Kampf |
|
| Redazionale (2005) |
|
| Turconi (1984) |
|
| Pipino (1984) |
|
| Camarda et al. (2013) |
|
| Marchesini M. (1999) |
|
| Voghera Museum of Natural Sciences (Arcallini find & donation) |
|
| Antofilli |
|
| Antofilli M. et al. (1983) |
|
| Antofilli |
- Porto Venere (Portovenere)
| Bianchi et al. (2016) |
|
| Antofilli et al. (1983) |
|
| Corrado Balestra - SEM-EDS 3M Ferrania ... |
|
| Antofilli |
- Castelvecchio di Rocca Barbena
| Pelloux (1926) +2 other references |
|
| Ravagnani (1974) |
|
| M.E. Ciriotti Probed 2005 |
|
| G. Armellino et al. (SV) |
|
| Notiziario di Mineralogia-Ferrania Club ... |
|
| Bracco (2006) |
|
| Bertolini A. Rivista Mineralogica ... |
|
| Del Caldo et al. (1973) |
|
| Gramaccioli et al. (1992) |
|
| Maida (2002) |
|
| Fabio Brignoli collection 1985 |
|
| Maida (2002) |
|
| Zambelli (1978) +2 other references |
|
| Maida (2002) |
|
| |
|
| Maida (2002) |
|
| Capitanio A. Il Ferro della Val di ... |
|
| Maida (2002) |
|
| Luigi Possenti Collection |
|
| Daniele Ravagnani - I giacimenti ... |
|
| Vergani et al. (2021) |
|
| Vergani et al. (2020) |
|
| Scaini (1970) +2 other references |
|
| Itinerari Mineralogici della Lombardia ... |
|
| Del Caldo et al. (1973) |
|
| Del Caldo et al. (1973) |
|
| Crali et al. (1975) |
|
| Vergani et al. (2020) |
|
| Vergani et al. (2020) |
|
| Battaini et al. (1942) |
|
| Vergani F. et al. (2020) |
|
| Alberto Vaghi collection |
|
| Bedognè et al. (2006) |
|
| Bedognè et al. (1993) |
|
| Bedognè et al. (2006) |
|
| AA. VV. |
|
| Ghizzoni et al. (2005) |
|
| Ghizzoni et al. (2005) |
|
| Ghizzoni et al. (2005) |
|
| Bedognè et al. (2006) |
|
| Bedognè et al. (2006) |
|
| Bedognè +2 other references |
|
| Bedognè et al. (2006) |
|
| Orlandi et al. (1) |
|
| P. Mattias- M. Guerra (2008) |
|
| Morici M. (1990) |
|
| Cingolani G. (1984) |
|
| Boni et al. (1976) |
|
| Giuseppe Pipino (1980) |
|
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| Cámara et al. (2014) +1 other reference |
|
| Martina (1967) +1 other reference |
|
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| Piccoli (2002) |
|
| Piccoli et al. (2007) |
|
| Piccoli G.C. (2002) +2 other references |
- Bagni di Vinadio Valley (Corborant Valley)
| Raccagni (2018) |
- Metropolitan City of Turin
| |
|
| Maletto G. et al. (1976) |
|
| Prete (1995) |
- Mussa Alp (Pian della Mussa; Piano della Mussa; La Mussa; Lamussa)
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| Brizio et al. (2010) |
|
| Jervis (1873) |
|
| Piccoli et al. (2007) |
|
| Campostrini (2001) |
|
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| Piccoli et al. (2007) |
|
| Bindi L. et al. (2016) |
|
| |
|
| Bruno Martini collection |
|
| Bianco et al. (2006) |
|
| forum.amiminerals.it (n.d.) |
|
| Gruppo Mineralogico Lombardo (10) |
|
| Boscardin M. (1990) |
|
| Jervis (1873) |
- Verbano-Cusio-Ossola Province
| Klemm et al. (2004) |
|
| Lapis (7/8) +1 other reference |
|
| Collected by Frank de Wit (1989-2000) |
|
| Blumenthal (1952) |
|
| Lapis (10) |
|
| Mattioli V. (1979) |
|
| Roggiani (1975) +1 other reference |
|
| Lapis (9) |
|
| Luigi Chiappino data |
- Metropolitan City of Cagliari
| Stara et al. (1993) |
|
| Stara et al. (1993) |
|
| Del Caldo et al. (1973) +1 other reference |
|
| Del Caldo et al. (1973) |
|
| Valera et al. (1965) |
|
| Palache et al. (1951) +1 other reference |
|
| Gamboni et al. (2006) |
|
| Lovisato (1903) |
|
| Orlandi et al. (2007) +1 other reference |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara |
|
| Stara P. (1996) |
|
| Stara et al. (1996) |
|
| Moldovan et al. (2013) |
|
| Stara et al. (1996) |
|
| P.Stara |
|
| Stara |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| 3 (3-1987) +4 other references |
|
| Brizzi G. et al. (1989) |
|
| Stara et al. (1996) |
|
| Stara et al. (1994) |
|
| Mariani P. et al. (1975) |
|
| Stara et al. (1996) |
|
| Fernando Caboni et al. (2024) |
|
| Fernando Caboni et al. (2024) |
|
| Ruggieri et al. (1) |
|
| Ruggieri et al. (1) |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Preite et al. (2007) |
|
| Del Caldo et al. (1973) +2 other references |
|
| Stara et al. (1996) |
|
| De Michele (1974) |
|
| Stara P. (1996) |
|
| Stara |
|
| Stara et al. (1996) |
|
| Stara et al. (1996) |
|
| Stara et al. (1993) |
|
| Stara et al. (1995) |
|
| Lovisato (1908) +3 other references |
|
| Cocco et al. (2022) |
|
| www.associazionemineralogicasarda.it (n.d.) |
|
| Rivista Mineralogica Italiana 3/1992-"I minerali del giacimento di Monte Tamara (Nuxis) |
|
| Stara et al. (1993) |
|
| Stara et al. (1993) |
|
| Stara et al. (1993) |
|
| Stara et al. (1993) |
|
| Stara et al. (1993) |
|
| Stara et al. (1996) |
|
| [AmMin 85:1563] |
- Metropolitan City of Messina
- Eolie Islands (Aeolian Islands)
| Campostrini et al. (2011) |
|
| Musumeci (1958) +2 other references |
|
| Triscari (1985) |
|
| La Valle (1898) |
- Trentino-Alto Adige (Trentino-South Tyrol)
- Burggrafenamt (Burgraviato)
- Moos in Passeier (Moso in Passiria)
| Exel (1987) |
|
| Exel (1987) +6 other references |
|
| Exel (1987) +1 other reference |
|
| Exel (1987) |
|
| - (2023) |
|
| Exel (1987) |
|
| Exel (1987) |
- Sarentine Alps (Sarntal Alps)
| Exel (1987) |
- Überetsch-Unterland (Oltradige-Bassa Atesina)
| Niedermayr (2007) +1 other reference |
|
| Niedermayr (2007) +2 other references |
|
| Exel (1987) +1 other reference |
|
| Exel (1987) |
- Wipptal (Alta Vall'Isarco)
| Exel (1987) |
- Trento Province (Trentino)
| Daniele Ravagnani - I giacimenti ... |
|
| Daniele Ravagnani - Giacimenti ... |
|
| Exel (1987) |
|
| Bortolozzi (n.d.) |
|
| Bortolozzi (n.d.) |
|
| Forenza et al. (2005) |
|
| Exel (1987) |
|
| Exel (1987) +1 other reference |
|
| Ferretti P. et al. (Lavis, Trentino-Alto Adige) |
|
| Canal et al. (2012) |
|
| Murara G. (1966) |
|
| Exel (1987) |
|
| Vecchi et al. (2013) |
|
| Boscardin (1973) |
|
| Daniele Ravagnani - I giacimenti ... |
|
| Paolo Gasparetto et al. (2014) |
|
| M. Boscardin: Località mineralogiche consigliate: Miniera di Vignola in Valsugana (Trento) |
|
| Fabio Tosato +3 other references |
|
| doi:10.3390/min8060248 +2 other references |
|
| De Michele (1974) |
|
| I minerali dell' area delle Terme di ... |
|
| Marinai |
|
| "Lithothek der Münchener Micromounter" ... |
- Montoccoli-Castellaccia area (Fosso Zanca-Filone di Montoccoli area; Zanca Valley mines)
| Bazzoni C. et al. (2011) |
|
| Bazzoni C. et al. (2011) |
|
| Brizzi G. et al. (GR) |
|
| Sabelli C. et al. (GR) |
|
| Bazzoni et al. (2007) |
- Valpiana - Castello della Marsiliana area slag sites
| "Lithothek der Münchener Micromounter" ... |
|
| Jansen et al. (1998) |
|
| Del Caldo et al. (1973) |
|
| Bazzoni C. et al. +1 other reference |
|
| Batacchi et al. (2011) |
|
| Bazzoni et al. (2001) |
|
| A.Costantini (2015) |
|
| Rivista Mineralogica Italiana (1) |
|
| Dini et al. (2013) |
|
| Nannoni R. et al. (2012) |
|
| Int. Assoc. of Collectors of Slag Minerals (2) |
|
| vom Rath G. (1868) |
|
| De Michele (1974) +1 other reference |
|
| |
|
| F. Millosevich (1914) |
|
| Ciampalini et al. (2010) |
|
| Sammartino (2007) +1 other reference |
|
| Marco Bonifazi collection |
|
| Marinai V. et al. (Livorno) |
- Rio Ardenza (Rio Popogna)
| Bonifazi (2020) +1 other reference |
|
| Bonifazi (2021) |
|
| Franzini et al. (1992) +2 other references |
|
| Gilliéron (1959) +3 other references |
|
| F. Gillieron |
- Rialbano Mine (Rio Albano Mine)
| N. Calanchi |
|
| G. Tanelli et al. (Isola d’Elba) |
|
| Bonifazi (2020) |
|
| Jansen et al. (1998) |
|
| Luigi Chiappino data |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| Orlandi et al. (1996) +1 other reference |
|
| Orlandi et al. (2004) |
|
| D'Achiardi A. |
|
| Orlandi et al. (1980) +2 other references |
- Colonnata quarrying basin
| Morino (2017) |
|
| Franzini et al. (1982) |
|
| Orlandi et al. (1980) +4 other references |
- Miseglia quarrying basin (Miseglia-Fantiscritti quarrying basin; Fantiscritti quarrying basin)
| Chollet Pascal specimen & photo +2 other references |
|
| Orlandi et al. (1980) +1 other reference |
|
| Morino (2017) |
|
| Marco Albertazzi Collection |
|
| Marco Albertazzi Collection |
|
| Orlandi et al. (2009) |
|
| Orlandi et al. (2009) |
|
| Orlandi et al. (1994) +2 other references |
|
| Brizzi (1986) |
|
| D’Achiardi (1872-73) +3 other references |
|
| Mascaro et al. (1991) |
|
| Uzielli (1877) +1 other reference |
|
| Orlandi et al. (2009) |
- Canale del Fondone quarrying basin (Fondone quarrying basin)
| Pelloux (1929) +2 other references |
|
| Orlandi et al. (1989) +2 other references |
|
| Orlandi P. +1 other reference |
|
| Orlandi et al. (2002) |
- Metropolitan City of Florence
| Betti C. (2000) |
|
| · Betti et al. (2017) |
|
| Betti et al. (2017) |
|
| Betti C. (2000) |
|
| Brizzi G. et al. (FI) |
|
| Brizzi G. et al. (FI) |
|
| Brizzi G. et al. (FI) |
|
| · Betti et al. (2017) |
|
| · Betti et al. (2017) |
|
| Orlandi P. (2005) |
|
| Nannoni |
- Castelnuovo di Val di Cecina
| Brizzi et al. (3) |
|
| Orlandi P. (2005) |
- Montecatini Val di Cecina
| Terenzi A. et al. (PI) |
|
| Nannoni |
|
| Orlandi P. (2005) |
|
| D'Orazio et al. (2024) |
|
| D'Orazio et al. (2024) |
|
| Brizzi G. et al. (1996) |
|
| Brizzi G. et al. (1986) +1 other reference |
|
| C. Betti et al. (1999) |
|
| Nimis et al. (2011) |
|
| forum.amiminerals.it (n.d.) |
|
| |
- San Rocco di Tretto (San Rocco)
| Pegoraro et al. (1997) +3 other references |
|
| Ferialdi et al. (2003) |
|
| Pegoraro S. et al. (2009) |
|
| Boscardin M. et al. (Vicenza) |
|
| Saccardo D. et al. (Torrebelvicino, Vicenza) |
- Monte Naro - Riolo Valley side
| Matteo Chineletto |
|
| Boscardin et al. (2011) +1 other reference |
|
| Zordan et al. (2001) +2 other references |
|
Jamaica | |
|
| Copper Handbook 1911 |
|
Japan | |
|
| Suzuki et al. (1976) +1 other reference |
|
| Sadanaga et al. (1974) |
|
| Sadanaga et al. (1974) |
|
| Okamoto +3 other references |
|
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
|
| Dr. Matsuo Nambu collection (curated at Geological Survey of Japan) |
|
| Journal of the Faculty of Science et al. (2) |
|
| Journal of the Faculty of Science et al. (2) |
|
| 島倉広至 et al. (2015) |
|
| Myokensanroku-iseki-chosa-kai (1994) |
|
| Hiroaki Tano's collection |
|
| Copper Handbook 1911 |
|
| Sadanaga et al. (1974) |
|
| Petrov (n.d.) |
|
| Sadanaga et al. (1974) |
|
| Radioactive minerals from Shin-mikawa ... |
|
| Watanabe et al. (2003) |
|
| Petrov (n.d.) |
|
| Sadanaga et al. (1974) +1 other reference |
|
| Yamada (2004) |
|
| Copper Handbook 1911 |
|
| Petrov (n.d.) |
|
| Petrov (n.d.) |
|
| Nagashima et al. (2021) |
|
| Wada (1904) +2 other references |
|
| Uehara et al. (2014) |
|
Kazakhstan | |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Kalinin et al. (2018) |
|
| Pavel.M. Kartashov (n.d.) |
|
| Lobanov et al. (2012) |
|
| Pavel.M. Kartashov (n.d.) |
|
| Litvinovich et al. (1960) |
|
| Singer et al. (2008) |
|
| Jeremolenko (2002) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
- Gul'shad (Gul'shat; Gul'sad)
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Evseev (1995) +1 other reference |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Anthony et al. (2000) |
|
| Singer et al. (2008) |
|
| Pekov (1998) |
|
| Singer et al. (2008) |
|
| - (2005) |
|
| Min News 20:6 pp3 |
|
Kyrgyzstan | |
|
| Filippov et al. (2013) |
|
| Kolesar et al. (1993) |
|
| Kolesar et al. (1993) |
|
| www.imgeology.com (2013) |
|
| Fleischer et al. (1982) |
|
| Pavel.M. Kartashov (n.d.) |
|
| Baocheng Fan et al. (2014) |
|
Laos | |
|
| De Ascenção Guedes et al. (2015) |
|
| Niedermayr et al. (2012) |
|
Luxembourg | |
|
| Simon Philippo analysis (to be published) +1 other reference |
|
Madagascar | |
|
| Lacroix (1899) |
|
| Behier (1960) |
|
| Behier (1963) |
|
| Behier (1963) |
|
| Behier (1963) |
|
| Behier (1960) |
|
| Behier (1960) |
|
| Behier (1963) |
|
| Behier (1963) |
|
| Behier (1963) |
|
| Behier (1963) |
|
Malaysia | |
|
| Hoe et al. (2003) |
|
| Teh (1981) |
|
| Singer et al. (2008) |
|
Mali | |
|
| Maiga et al. (2003) |
|
| Masurel et al. (2017) |
|
Mauritania | |
|
| Marsh et al. (2015) |
|
| Marsh et al. (2015) |
|
| Marsh et al. (2015) |
- Akjoujt (Akjout; Fort Repoux)
| Marsh et al. (2015) |
|
| Marsh et al. (2015) |
|
| Kolb et al. (2006) |
|
| Marsh et al. (2015) |
|
Mexico | |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panzcer (1987) |
|
| Panczner (1987) |
|
| Singer et al. (2008) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Copper Handbook 1911 |
|
| Panczner (1987) |
|
| Panczner (1987) |
- Aquiles Serdán Municipality
- Santa Eulalia Mining District
| Panczner (1987) |
- Andrés del Rio mining district
| Wilson et al. (1986) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- Cusihuiriáchic Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Singer et al. (2008) |
- Hidalgo del Parral Municipality
| Panczner (1987) |
- Manuel Benavides Municipality
| Bill Dameron photo |
|
| Gonzalez-Reyna 1956 +1 other reference |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- Municipio Valle de Rosario
| Rob Lavinsky (ex. Sam Nasser specimen) |
|
| Querol +2 other references |
|
| Megaw (2023) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- La Encantada mining district
| Panczner (1987) |
- Sierra Mojada Municipality
| Panczner (1987) |
|
| Dana 7. +2 other references |
|
| Megaw (2023) |
- Santa María del Oro (El Oro)
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Rocks & Min.: 56:247. +1 other reference |
|
| Thomas P. Moore (2008) |
- Pánuco de Coronado Municipality
| Panczner (1987) |
- San Pedro del Gallo Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- San Luis de la Paz Municipality
| Panczner (1987) |
|
| Panczner (1987) |
- San Miguel de Allende Municipality
- Mineral de Puerto de Nieto (Puerto de Nieto; Puerto Nieto)
| Panczner (1987) |
- Eduardo Neri Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Dana 7:I:518. +1 other reference |
|
| Panczner (1987) |
- Chiquilistlán Municipality
| Panczner (1987) |
|
| Camprubí et al. (1) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Singer et al. (2008) |
- Catorce (Real de Catorce)
| Panczner (1987) |
- Cerro de San Pedro Municipality
- Cerro de San Pedro (San Pedro)
| Panczner (1987) |
|
| Panczner (1987) |
|
| Dr. Walter S. Bowser Collection |
- Villa de Ramos Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| |
|
| Copper Handbook 1911 |
|
| Espinosa Perea (1999) +1 other reference |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Pancnzer (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| |
|
| Braith et al. (2001) +1 other reference |
|
| Lapis 2001 (1) |
- Nacozari de García Municipality
| Panczner (1987) |
|
| |
|
| Valencia et al. (2006) +2 other references |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- Suaqui Grande Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- Concepción del Oro Municipality
| Panczner (1987) |
|
| Panczner (1987) |
|
| |
|
| Norman King and many dealers +1 other reference |
|
| Panczner (1987) |
|
| Stone +3 other references |
|
| Part I et al. (8) +1 other reference |
|
| Betts (n.d.) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Dana 7:II:954. +1 other reference |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
|
| Megaw (2023) |
- Noria de Ángeles Municipality
- Noria de Angeles (Noria de los Angeles)
| Panczner (1987) |
|
| Panczner (1987) |
|
| Panczner (1987) |
- San Martín-Sabinas District
| Megaw (2023) |
|
| Panczner (1987) |
|
Mongolia | |
|
| Soloviev et al. (2022) |
|
| Singer et al. (2008) |
- Tsagaan Suvarga ore field (Tsagaan Suvarguin ore field; Tsagan Suburga ore field)
| Weixuan Fang et al. (2007) |
|
| Petr Paulis (Bajan Mod) |
|
| Singer et al. (2008) |
|
| web.archive.org (n.d.) |
|
| web.archive.org (n.d.) |
|
| Davaasuren et al. (2016) +1 other reference |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Robin Grayson & Baatar Tumenbayar (2005) |
|
Morocco | |
- Béni Mellal-Khénifra Region
| King (n.d.) |
|
| Ibouh et al. (n.d.) |
|
| Fabre Minerals (see photo) |
|
| Jahn et al. (2003) |
|
| Jordi Fabre |
|
| Samaoui et al. (2024) |
|
| Rob Lavinsky (ex. Howard Belsky specimen) |
|
| Jébrak et al. (1998) |
|
| Fabre (n.d.) |
|
| Jébrak et al. (1998) |
|
| |
|
| Rob Lavinsky |
|
| Fabre (n.d.) |
|
| www.fabreminerals.com +1 other reference |
|
| Praszkier (2015) |
|
| Benchekroun et al. (2004) |
|
| ASLADAY et al. (1998) |
|
| Favreau (2005) |
- Bou Skour mining district
| |
|
| Georges Favreau collection |
|
| American Mineralogist 55:1448 |
|
| Georges Favreau collection |
|
| Georges Favreau collection |
|
| Favreau et al. (2006) |
|
| Favreau et al. (2006) |
|
| Favreau et al. (2006) |
|
| Favreau et al. (2006) |
|
| Asladay et al. (2012) |
|
| Jahn et al. (2003) |
|
| Jahn et al. (2003) |
|
| Alain Guillet collection |
|
| Ilmen et al. (2024) +1 other reference |
|
| Georges FAVREAU collection + EDX ... |
|
| Barral (2023) |
|
| Bouabdellah et al. (2009) |
- Jebilet Mtn (Djebilet Mtn)
| Essarraj et al. (2022) |
|
| Georges Favreau collection |
- Touissit-Bou Beker mining district
| Georges Favreau collection |
|
| |
|
| King (n.d.) |
|
| Rocks & Min.: 65:27. |
|
| King (n.d.) |
|
| Louis Verschuren Collection |
|
| Oummouch et al. (2017) |
|
| Fabre (n.d.) |
|
| El Basbas et al. (2011) +1 other reference |
|
Myanmar | |
|
| Aung Myo et al. (2019) |
|
| Sint et al. (2019) |
|
| Sint et al. (2019) |
|
| Sint et al. (2019) |
|
| Sint et al. (2019) |
|
| Sint et al. (2019) |
|
Namibia | |
|
| various added photos +1 other reference |
|
| Donald Boyack (2010) |
|
| Bezing et al. (2007) |
|
| Bezing et al. (2007) +1 other reference |
|
| Jahn et al. (2006) +1 other reference |
|
| ... +1 other reference |
|
| von Bezing (2007) |
|
| |
- Mesopotamia copper valley
| Niedermayr (2001) +1 other reference |
|
| Bowell et al. (2013) |
|
| von Bezing et al. (2016) |
|
| Palfi (2005) +1 other reference |
|
| Brandstätter +4 other references |
|
| Cairncross et al. (1997) |
|
| Cairncross (1997) |
|
| Mason (1976) |
|
| Thomson (1917) +2 other references |
|
| www.namibiaminerals.com (2003) |
- Grootfontein Constituency
| Chetty et al. (2000) |
|
| Bezing et al. (2007) |
|
| von Bezing (2007) |
|
| Dunn (1991) +1 other reference |
|
| von Bezing (2007) |
|
| von Bezing (2007) |
|
| Singer et al. (2008) |
|
| Cairncross et al. (2012) |
|
| von Bezing (2007) |
|
| von Bezing (2007) |
|
| von Bezing (2007) +1 other reference |
|
New Zealand | |
|
| Moore et al. (1985) |
|
| Collection of RJ Martin |
|
| Becker et al. (2000) |
- Manawatu-Whanganui Region
| Christine Johnson comm. 2006 |
|
| Railton et al. (1990) |
|
| Railton et al. (1990) |
|
| Railton et al. (1990) |
|
| Collection of RJ Martin |
|
| Courtney et al. (1990) |
- Thames-Coromandel District
| Railton et al. (1990) |
|
| 7 +1 other reference |
|
| Collection of RJ Martin |
|
| Railton et al. (1990) |
|
Nigeria | |
|
| Ezepue (1984) |
|
North Macedonia | |
|
| Serafimovski et al. (2015) +1 other reference |
|
| Boev et al. (1993) |
- Kratovo-Zletovo ore district
| Serafimovski et al. (2003) +2 other references |
|
| Singer et al. (2008) +3 other references |
|
| Serafimovski et al. (2016) |
|
Norway | |
|
| Holm (1824) |
|
| Holm (1824) |
|
| Nordrum (2012) |
|
| Burvald (1983) |
|
| Collected by Peter Andresen 2009. |
|
| Nordrum (2002) |
|
| Juul Nilsen (1993) |
|
| Raade (1995) |
|
| Knut Edvard Larsen collection # MM-3413 |
|
| Peter Andresen collection. |
|
| Juul Nilsen (1993) |
|
| Goldschmidt (1911) +1 other reference |
|
| Stensrud (2004) |
|
| Raade (1995) |
|
| Peter Andresen collection. |
|
| Neumann (1985) |
|
| Vogt (1908) |
|
| Geological Survey of Norway. The Ore ... |
|
| Solnørdal (2001) |
|
| Rune S. Selbekk (2010) |
|
| Nordrum et al. (2006) |
|
| Vogt (1892) |
|
| Knut Eldjarn Collection |
|
| Goldschmidt (1911) |
|
| Sørlie (2003) |
|
| Cook et al. (2008) |
|
| Barentsburg Pomor museum collection |
|
| Kjærnet (2000) |
|
| Larsen et al. (2019) |
|
| Knut Edvard Larsen collection # MM-502 ( Visual id) |
- Dalane copper and silver mines
| Sverdrup et al. (1967) |
|
| Raade (1966) |
|
| |
|
| Neumann (1985) |
|
| Knut Edvard Larsen collection (# 166) |
|
| Knut Edvard Larsen observation in field ... |
|
| Nordrum (2008) |
|
| Rune S. Selbekk (2010) |
|
| P. Andresen (collected, August 2003) |
|
| Rø (2017) |
|
| Witsø (1994) |
|
| Witsø (1994) |
|
| Witsø (1995) |
|
| Jørgensen et al. (2001) |
|
| Jørgensen et al. (2001) |
|
| Rø et al. (1995, June 27th) |
|
| Neumann (1985) |
|
| Peter Andresen collection. |
|
| Goldschmidt (1911) |
|
| Frigstad et al. (1969) +1 other reference |
|
| Folvik (2005) |
|
| Rune Fjellvang observation in field ... |
|
| Berg (1994) |
|
| Kamphaug (1986) |
|
| Karstensen (2009) |
|
Oman | |
|
| Rajendran et al. (2013) |
|
Pakistan | |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Ahmed et al. (2017) |
|
| Jose Zendrera Collection |
|
Papua New Guinea | |
|
| Noku et al. (2012) |
|
| aufa.tripod.com (n.d.) |
- East New Britain Province
| Seccombe et al. (2005) |
- Eastern Highlands Province
| Espi et al. (2007) |
|
| Traill (1961) |
|
| Singer et al. (2008) |
|
| Singer et al. (2005) |
|
| Espi et al. (2002) +1 other reference |
|
| Mine geologist |
|
Paraguay | |
|
| USGS Proffesional Paper 327 |
|
Peru | |
|
| |
|
| Singer et al. (2008) |
|
| - (n.d.) |
|
| Crespo +4 other references |
|
| Alfonso et al. (2023) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Lewis Jr (1964) |
|
| Econ Geol (1981) |
|
| Singer et al. (2008) |
|
| Petersen (1970) |
|
| Petersen (1970) |
|
| Petersen (1970) |
|
| Petersen (1970) |
|
| Hulse (2015) |
- General Sánchez Cerro Province
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Ascencios (1966) |
|
| Singer et al. (2008) |
|
Philippines | |
|
| Natural History Museum Vienna ore ... |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
- Cordillera Administrative Region
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
- Surigao del Norte Province
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2005) |
|
| Singer et al. (2008) |
- Negros Occidental Province
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| Herschel Ward specimens |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
|
| South-East Asian Oil |
|
Poland | |
- Lesser Poland Voivodeship
| Wojciechowski J. 1955: O żyłach ... |
|
| Paulo A. 1970: Mineralizacja ... |
- Lower Silesian Voivodeship
- Gmina Warta Bolesławiecka
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Siuda (2001) |
|
| Lis et al. (1986) |
|
| Paulo A. 1970: Minerały niklu i bizmutu w żyłach kruszcowych okolicy Chełmca (Góry Kaczawskie, Dolny Śląsk) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Domań +2 other references |
|
| Mochnacka et al. (2012) |
|
| Lis et al. (1986) |
|
| Pieczka et al. (1988) |
|
| Lis et al. (1986) |
|
| Mochnacka et al. (2015) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Eligiusz Szełęg collection (SEM/EDS identification) +1 other reference |
|
| pl.wikipedia.org (2006) |
|
| Piestrzyński (Main Ed.) |
|
| Piestrzyński et al. (2012) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Siuda (2014) |
|
| Lis et al. (1986) |
|
| Lis et al. (1986) |
|
| Wyżykowski J. 1979 - Rudy miedzi in ... |
|
| Breslau. P. 1-285 +1 other reference |
|
| Breslau. P. 1-285. +2 other references |
|
| Lis et al. (1986) |
|
| Wyżykowski J. 1979 - Rudy miedzi in ... |
- Świętokrzyskie Voivodeship
| Ciurej et al. (2021) +1 other reference |
|
| Rubinowski (1955) |
|
| Eligiusz Szełęg collection |
|
Portugal | |
|
| |
- Almodôvar e Graça dos Padrões
| Rui Nunes collection |
|
| |
|
| Rui Nunes and Pedro Alves collections +1 other reference |
|
| Alves (n.d.) |
|
| Alves (n.d.) |
|
| Pedro Alves collection. |
|
| Alves (n.d.) |
|
| LNEG Siorminp database |
|
| LNEG - Siorminp database information |
|
| Rui Nunes collection. |
|
| LNEG Siorminp database |
|
| |
|
| LNEG |
|
| LNEG - Laboratório Nacional de Energia ... |
|
| Silva (1949) |
|
| Júlio António Vieira da Silva Pinto et al. (arquivo da Repartição de Minas) |
|
| LNEG - Laboratório Nacional de Energia ... |
|
| Pedro Alves collection and analytical ... |
|
| Ivo Rodrigues and Hugo Lobo's mineral ... |
- Ferreira do Alentejo e Canhestros
| Alves (n.d.) |
|
| Alves et al. (2017) |
|
| Pedro Alves collection and analytical ... |
- Vila Nova de São Bento e Vale de Vargo
| Alves (n.d.) |
|
| Ricardo Silva's Collection |
|
| Ricardo Silva's personal collection |
|
| Pedro Alves collection and analytical ... |
|
| Alves (n.d.) |
|
| |
- União de Freguesias de Estreito e Vilar Barroco
| Martins da Pedra collection |
|
| |
|
| Martins da Pedra collection |
|
| Martins da Pedra collection |
- Alandroal (Nossa Senhora da Conceição), São Brás dos Matos (Mina do Bugalho) e Juromenha (Nossa Senhora do Loreto)
| Rui Nunes collection |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 1744Cu) |
- Nossa Senhora da Tourega e Nossa Senhora de Guadalupe
| Alexandre Pedroso field observations (August 2017) |
|
| Rui Nunes collection |
- Nossa Senhora da Vila, Nossa Senhora do Bispo e Silveiras
| Field observations (Pedro Alves, August 2017) |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 1721Cu) |
|
| Pedro Alves collection and analytical ... |
- Nossa Senhora da Conceição e São Bartolomeu
| Rui Nunes collection |
|
| Pedro Alves collection and analytical ... |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 769Cu) |
|
| |
|
| |
|
| Alves (n.d.) |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 1819Cu) |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 1704Cu) |
|
| LNEG - Laboratório Nacional de Energia e Geologia (Siorminp ID 2264Cu) |
- Torre de Terrenho, Terrenho e Sebadelhe da Serra
| LNEG - Laboratório Nacional de Energia ... |
- Santa Maria e São Pedro e Sobral da Lagoa
| LNEG - Laboratório Nacional de Energia ... |
|
| LNEG Siorminp database |
|
| LNEG - Laboratório Nacional de Energia ... |
|
| LNEG - Laboratório Nacional de Energia ... |
|
| LNEG - Laboratório Nacional de Energia ... |
- Alcácer do Sal e Santa Susana
| Field observations (Pedro Alves, 2009) |
|
| Pedro Alves self-colected |
|
| LNEG database |
- Santiago do Cacém, Santa Cruz e São Bartolomeu da Serra
| LNEG database |
|
| LNEG database |
|
| LNEG database |
|
| LNEG database |
|
| LNEG database |
|
| Alves (n.d.) |
|
| Alves (2017) |
- U.F S.J.da Pesqueira e Várzea de Trevões
| |
|
| J.M. Cotelo neiva (1944) |
|
Republic of the Congo | |
|
| Demetrius Pohl |
|
| Demetrius Pohl |
|
| Demetrius Pohl +1 other reference |
|
Romania | |
|
| Rădulescu Dan |
|
| Szakáll (2002) |
|
| Szakáll (2002) |
|
| Canadian Mineralogist 40:1693-1703. |
|
| www.minerals-of-the carpathians.eu (2009) |
|
| Shimizu et al. (1998) |
|
| Canadian Mineralogist Vol. 40 (2002) |
|
| Onac et al. (2002) |
|
| Onac et al. (2002) |
|
| Palache et al. (1951) |
|
| minerals-of-the-carpathians.eu (2008) |
|
| Szakáll (2002) |
|
| Szakáll (2002) |
|
| Acta Mineralogica Petrographica |
|
| Szakáll et al. (2010) |
|
| 1-Rădulescu Dan |
|
| Udubaşa S.S. (2004) |
|
Russia | |
- Adygea (Republic of Adygea)
| Pekov I.V. et al. (2010) +1 other reference |
- Krasnoshchyokovsky District
| Gusev A.I. Geochemical features of the gold mineralization of the Murzinsky ore field of the Gorny Altai // Successes of modern natural science. – 2014. – № 9 (2) +3 other references |
|
| Palache et al. (1951) |
|
| Pavel.M. Kartashov (n.d.) +2 other references |
|
| Lapis Nov -94 p48-49 |
|
| Potseluev et al. (2006) |
|
| be.sci-lib.com (n.d.) |
|
| Michurin et al. (2024) |
|
| Kontar E.S. Geological and industrial types of deposits of copper et al. (Anniversary - p. 99 -100) |
|
| Mironov et al. (2008) |
|
| Kasatkin et al. (2022) |
|
| Christine Iacobuzio-Donahue and Quebul ... |
|
| Plotinskaya et al. (2018) |
|
| Alexei Kuznetsov data |
- Bereznyakovskoe ore field
| Plotinskaya et al. (2009) |
|
| Igor Savin data |
- Ufaley District (Ufalei District)
| Local geolofist S.V.Kolesnichenko data |
- Chukotka Autonomous Okrug
| Savva et al. (2011) |
|
| Singer et al. (2008) |
|
| Singer et al. (2008) |
- Chaunskii District (Chaun)
| Pekov et al. (2016) |
|
| Sidorov et al. (2017, August) |
- Ust-Bolsheretsky District
| Novakov et al. (2021) |
|
| Lopatkin Oleg |
- Khakassia (Republic of Khakassia)
| Kassandrov et al. (2009) |
|
| Kassandrov et al. (2009) |
- Khanty-Mansi Autonomous Okrug
| Burlakov (1995) |
|
| Bukanov et al. (2012) |
|
| Kuznetsov et al. (2012, January) |
- Taymyrskiy Autonomous Okrug
| Pavel.M. Kartashov (n.d.) |
|
| Singer et al. (2008) |
|
| Savva et al. (2011) |
|
| Volkov et al. (2008) |
|
| Savva et al. (2007) |
|
| |
- Pai-Khoi Range (Paikhoi; Pay Khoy)
- Middle Silova-Yakha River
| Koval'chuk et al. (2008) |
|
| Rakhimov et al. (2022) |
- Dalnegorsk Urban District
| Grant et al. (2001) |
|
| Vasilenko (2001) |
- Kavalerovo mining district
| Кокорин et al. (2000) |
|
| Frank‑Kamenetskaya et al. (2021) |
- Pitkyaranta mining district (Pitkäranta mining district)
| Ivashchenko et al. (2011, September) |
|
| [World of Stones 12:49] |
|
| USGS Open File Report 2005-1252 p25 |
|
| Nenasheva et al. (2011) |
|
| Nokleberg (2005) |
|
| Kolev et al. (2010) |
|
| Palache et al. (1951) |
|
| Fersman Mineralogical Museum |
|
| Kievlenko E.V. (1983) |
|
| Collection of Naturhistoriska Riksmuseet i Stockholm (The Natural History Museum) |
|
| Kasatkin et al. (2021) |
|
| |
- Medniy village ("Copper village")
| Mishainik |
|
| Pekov (1998) |
|
| Kuzhuget et al. (2022) |
|
| Kuzhuget et al. (2015) |
|
| Kuzhuget et al. (2022) |
|
| R. V. Kuzhuget et al. (2017) |
|
| [Mineralien Welt 6/92:62] |
- Yamalo-Nenets Autonomous Okrug
| Vikentyev et al. (2023) |
- Gazimuro-Zavodsky District
| Svetlitskaya et al. (2022) |
|
| Redin et al. (2020) |
- Kodar–Udokan ore district
| Gongalsky et al. (2019) |
|
| Gongalsky et al. (2024) |
|
Saudi Arabia | |
|
| Charles W. Smith et al. (1976) |
|
| Hakim et al. (1992) |
|
Serbia | |
|
| Slobodan A. Radosavljević et al. (2013) |
|
| Radosavljevic-Mihajlovic et al. (2007) |
|
| Radosavljević-Mihajlović et al. (2017) |
|
| Albrecht von Quadt et al. (2007) |
|
| Albrecht von Quadt et al. (2007) |
|
| Jovanović (2009) |
|
| Ministry of Mining and Energy (2002) |
|
| Ministry of Mining and Energy (2002) |
|
Slovakia | |
|
| Grecula (1995) |
|
| Andráš et al. (2009) |
|
| Kodě +2 other references |
|
| Koděra (1986) |
|
| Števko et al. (2016) |
|
| 70. +1 other reference |
|
| Polák Ľ. & Ferenc Š (2016) |
|
| Palache et al. (1951) |
|
| Koděra (1986) |
|
| Koděra (1986) |
- Banská Štiavnica District
| |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Hak J. & Losert J. |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Luptáková J. |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2022) |
|
| Kodě +1 other reference |
|
| Števko M. et al. (in Slovak) |
|
| Števko M. et al. (Slovenská republika) |
|
| Martin Števko-unpublished |
|
| Ďuďa (1993) |
|
| Koděra (1986) |
|
| Sejkora J. |
|
| Háber et al. (SGR) |
|
| Háber M. (1969) |
|
| Háber et al. (SGR) |
|
| Koděra (1986) |
|
| Pauliš P. |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Zipser (1817) |
|
| Koděra (1986) |
|
| Koděra (1986) |
- Prakovce Fe-Cu mining district
| Grecula (1995) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2015) |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Pauliš P. |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Sejkora et al. (2024) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2022) |
|
| Martin Števko-unpublished |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Marek Szabó Dósza-visual ... |
|
| Martin Števko-unpublished |
|
| Števko M. (2022) |
|
| Martin Števko |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2022) |
|
| Števko M. et al. (in Slovak) |
|
| Grecula (1995) |
|
| Grecula (1995) |
|
| Pauliš P. |
|
| Grecula (1995) |
|
| Grecula (1995) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2022) |
|
| Števko et al. (2009) |
|
| Koděra (1986) |
|
| Grecula (1995) |
|
| Grecula (1995) |
|
| Martin Števko |
|
| Števko M. (2022) |
|
| Koděra (1986) |
- Spišská Nová Ves District
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Števko M. (2022) |
|
| Števko M. et al. (2017) +1 other reference |
|
| Julia Herrmann et al. (2018) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Ďuďa R. |
|
| Uher P. & Ozdín D. (Tribeč, Slovensko) |
|
| Ferenc et al. (2001) |
|
| Ferenc et al. (2020) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Pauliš |
|
| Koděra (1986) |
|
| Roman Gramblička collection |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Daniel J. et al. (2005) |
- Liptovský Mikuláš District
| Pauliš |
|
| Koděra (1986) |
|
| Chovan et al. (1995) |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
| Rojkovič I. (1998) |
|
| Ozdin D. |
|
| Koděra (1986) |
|
| Koděra (1986) |
|
Slovenia | |
|
| Scopolia - Journal of the Slovenian ... |
|
| Scopolia - Journal of the Slovenian ... |
|
| Žorž et al. (2002) |
|
South Africa | |
- Chris Hani District Municipality
- Inxuba Yethemba Local Municipality
| Cairncross et al. (1995) |
|
| Cairncross et al. (1995) |
- Nelson Mandela Bay Metropolitan Municipality
| Personally collected by Reinhardt van ... |
- City of Tshwane Metropolitan Municipality
| Dana 6:A1:7 +2 other references |
|
| Rocks & Minerals 83:5 pp 410-421 +1 other reference |
- uMzinyathi District Municipality
| Cairncross et al. (1995) |
- Mopani District Municipality
- Ba-Phalaborwa Local Municipality
- Consolidated Murchison Mines
| Cairncross et al. (1995) |
|
| Chris DeGrave Collection |
- Sekhukhune District Municipality
- Ephraim Mogale Local Municipality
| Robert O. Meyer collection +2 other references |
|
| Cairncross et al. (1995) |
|
| C. Lemanski collection (7950CL & 7955CL) |
- Vhembe District Municipality
- Musina Local Municipality
| Cairncross (1991) +1 other reference |
|
| Cairncross et al. (1995) |
- Waterberg District Municipality
- Bela-Bela Local Municipality
| Atanasova et al. (2016) |
- Ehlanzeni District Municipality
- Mbombela Local Municipality
| Cairncross et al. (1995) |
- Thaba Chweu Local Municipality
| Cairncross et al. (1995) |
|
| Arthur L. Flagg Collection No. 3373 |
|
| Donald Volkman Personal Collection |
- Nkangala District Municipality
- Thembisile Hani Local Municipality
| Cairncross (2004) |
- Victor Khanye Local Municipality
| Cairncross et al. (1995) |
|
| SAMS (South African Micromount Society) |
|
| SAMS (South African Micromount Society) |
- Bojanala Platinum District Municipality
- Madibeng Local Municipality
| Windisch et al. (1999) |
- Rustenburg Local Municipality
| Wight (2002) |
- Dr Kenneth Kaunda District Municipality
- JB Marks Local Municipality
| Cairncross et al. (1995) |
|
| Cairncross et al. (1995) |
- John Taolo Gaetsewe District Municipality
- Joe Morolong Local Municipality
| Cairncross et al. (1995) +1 other reference |
|
| |
|
| Cairncross et al. (1995) |
- Namakwa District Municipality
- Khâi-Ma Local Municipality
| Cairncross et al. (1995) |
|
| Cairncross (2004) +1 other reference |
|
| Ronaldson (1905) |
|
South Korea | |
|
| Tsuda (1969) |
|
| Ryoo et al. (2014) |
|
Spain | |
|
| Calvo (2012) |
|
| Joan Rosell (2022) |
|
| Navarro et al. (2009) |
|
| Viñals et al. (2008) |
|
| Calvo (2012) |
|
| Georges FAVREAU collection & EDX ... |
|
| |
|
| Breithaupt (1852) |
|
| Valladares et al. (2021) |
|
| Georges Favreau collection |
|
| VIÑALS et al. (2004) |
|
| José Miguel Sola Fernández and group. |
|
| Weppe et al. (Almería) |
|
| A. Arribas et al. (2005) |
|
| G. Leal et al. (2005) |
|
| Calvo Rebollar (2012) |
|
| Jose Miguel Sola collection |
|
| MINDAT Sierra de Baza (Mineralogist Group of Granada) |
|
| CASTRO et al. (2022) |
|
| Calvo Sevillano et al. (2011) |
|
| Calvo (2012) |
|
| Carmona Ruiz et al. (2018) |
|
| Carmona Ruiz +2 other references |
|
| |
|
| Gómez F. & Sola J.M. (2000) |
|
| José Miguel Sola |
|
| Calvo (2012) |
|
| Mata Perelló (1991) |
|
| Solá |
|
| M. Ruiz Montes +1 other reference |
|
| Pierre Rondelez |
|
| J. Gröbner and M. A. Fernández Périz (2006) |
|
| Calvo Rebollar (2009) |
|
| García et al. (2003) |
|
| Fabre (n.d.) |
|
| Jordi Fabre (Fabre Minerals) |
|
| Garcia (1996) |
|
| Colectivo Arrayanes et al. (2008) |
|
| Romero Silva et al. (2013) |
|
| Romero Silva (2003) |
- Este (District 2; Málaga-Este)
| Romero Silva JC (2003) |
|
| Geological Museum of Malaga |
|
| Romero Silva JC (2003) |
|
| Romero Silva JC (2003) |
|
| Calvo (2012) |
|
| Calvo (2008) |
|
| Joan Rosell (2022) |
|
| Calvo (2008) |
|
| Calvo (2012) |
|
| Calvo (2008) |
|
| Calvo (2008) |
|
| Calvo (2008) |
|
| Analyzed by XRD by Dr. Joan Vinals (Barcelona) +1 other reference |
|
| Calvo (2008) |
|
| Calvo (2008) |
|
| 2009 |
|
| Gutiérrez et al. (2000) |
|
| Manuel Gutierrez Claverol y Carlos ... |
|
| Calvo (2012) |
|
| José Ramón García |
|
| Manuel Gutierrez Claverol et al. (2009) |
|
| Manuel Gutierrez Claverol y Carlos ... |
|
| López (n.d.) |
|
| Calvo (2012) |
|
| Joaquin Puga collection |
|
| IGLESIAS et al. (1994) |
|
| Manuel Gutierrez Claverol et al. (2009) |
|
| Calvo (2012) |
|
| Calvo (2012) |
|
| Martins da Pedra. |
- Colom island (Illa d‘en Colom)
| Hunt et al. (2014) |
|
| Crespí et al. (1998) |
- Legutio-Villarreal de Álava
| Calvo et al. (1993) |
|
| mti-minas-euskadi.blogspot.com (n.d.) |
|
| Calvo (2012) |
|
| Calvo (2012) |
|
| Díaz de Baranda et al. (2009) |
|
| Mineralogical Record (3) |
|
| Fabre (n.d.) |
|
| FMF Forum |
|
| |
- Aldeanueva de San Bartolomé
| Calvo (2015) |
|
| www.foro-minerales.com (n.d.) |
|
| info.igme.es (n.d.) |
|
| Henwood (1871) |
|
| Henwood (1871) |
|
| Henwood (1871) |
|
| Miguel Calvo Rebollar (2015) |
|
| J. A. Cendón collection |
|
| Calvo (2012) |
|
| Martínez i Cantó (2015) |
|
| Calvo et al. (2002) |
|
| Calvo (2012) |
|
| Ko Jansen |
|
| Calvo (2014) |
|
| Joan Font (2015) |
|
| Joan Rosell (2023) |
|
| Mata i Perelló (1990) |
- Vallès Occidental-Baix Llobregat
- Sant Cugat del Vallès-El Papiol
| Calvo (2012) |
|
| Mineralogistes de Catalunya (1997) |
|
| |
- Sant Fost de Campsentelles
| Ignacio Herrera +13 other references |
|
| Garrido et al. (2013) |
|
| |
|
| Joan Rosell (2021) |
|
| Mata i Perelló (1990) |
|
| |
|
| Joan Rosell et al. (2022) |
|
| Mata i Perelló (1990) |
|
| Mata i Perelló (1990) |
|
| José Zendrera inspection |
|
| Mata i Perelló (1990) +1 other reference |
|
| Calvo (2012) |
|
| Aufschluss 1975 (6) |
|
| Calvo Rebollar (2012) |
|
| |
|
| |
|
| Joan Rosell (02/2016) |
|
| J. Rosell (02/2016) |
|
| Aufschluss 1975 (6) |
|
| Joan Abella i Creus (2008) |
|
| Mata i Perelló (1990) |
|
| Rosell et al. (2017) |
|
| Jiménez R. et al. (2004) |
|
| Jiménez R. et al. (2004) |
|
| Jiménez R. et al. (2004) |
|
| Jordá (2008) |
|
| Jiménez Martínez |
|
| Jimenez R. et al. (2004) |
|
| Jiménez Martínez |
|
| Jiménez Martínez |
|
| Jiménez R. et al. (2004) |
- Gargantilla del Lozoya y Pinilla de Buitrago
| Jiménez Martínez et al. (2013) |
|
| Jimenez R. et al. (2004) |
|
| Geominero Museum of Madrid Collection |
|
| Tanago et al. (2012) |
- Loyozuela-Navas-Sieteiglesias
| www.foro-minerales.com (n.d.) |
|
| Geominero Museum of Madrid Collection |
|
| www.foro-minerales.com (n.d.) |
|
| Jiménez Martínez |
|
| www.mineralogia.es (n.d.) |
|
| Calvo (2012) |
|
| Calvo (2012) |
|
| Calvo Rebollar (2012) |
|
| Calvo (2012) |
|
| Minerales de la Región de Murcia +1 other reference |
|
| Luetcke (n.d.) |
|
| Calvo (2015) |
|
| Calvo (2012) +1 other reference |
|
| Sainz de Baranda et al. (2003) |
|
| Lapis 2002 (11) |
|
| Casanova Honrubia |
|
| Joan Vinals & Miguel Calvo (2007) |
|
| Casanova Honrubia |
|
| Calvo Rebollar (2012) |
|
| Casanova Honrubia |
|
| Juan Miguel Casanova |
|
| - (n.d.) +1 other reference |
|
| issuu.com (n.d.) +1 other reference |
- Vilamarxant (Villamarchante)
| Casanova Honrubia |
|
Sri Lanka | |
|
| Samarakoon et al. (2021) |
|
Sudan | |
|
| Jianyong Hu et al. (2011) |
|
| Adam et al. (2024) |
|
| Vail (1978) +1 other reference |
|
Sweden | |
|
| Öhman et al. (2000) |
|
| |
|
| Lorin et al. (2012) |
|
| |
|
| Shaikh-Persson-Sundberg-Wik: SGU Rep. ... |
|
| Swedish Nat.Musuem |
|
| The Swedish Museum of Natural History |
|
| Shaikh-Persson-Sundberg-Wik: SGU Rep. ... |
|
| Found and analyzed by Aksel Österlöf +1 other reference |
|
| |
|
| |
- Kiruna Town Mining District
| |
|
| Valentin Alain (2014) |
|
| Thorin (1989) +1 other reference |
|
| |
|
| Tegengren (1924) +1 other reference |
|
| Burke et al. (1990) |
|
| Gatedal (1997) |
|
| Jasinski (1983) |
|
| Canadian Mineralogist (1984) |
|
| |
|
| Wik et al. (1999) |
|
| Persson (1999) |
|
| Gatedal (n.d.) +1 other reference |
|
| Holtstam et al. (1999) |
|
| Holtstam et al. (1993) |
|
| Sandström et al. (2009) |
|
| Sandström et al. (2009) |
|
| Nysten (1984) |
|
| Persson (2006) |
|
| |
|
| Flink G. |
|
| Öhman et al. (2004) |
|
| Collection of Naturhistoriska Riksmuseet i Stockholm (The Natural History Museum) |
|
| |
|
| |
|
| SGU R&M 108 (2002) |
|
Switzerland | |
|
| Bächtiger (1963) +1 other reference |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Amstutz C. (1950) |
|
| Amstutz C. (1950) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) +1 other reference |
|
| Stalder et al. (1998) |
|
| Wenk et al. (2019) |
|
| Theobald (1866) +1 other reference |
|
| Cabalzar (1977) |
|
| Die Mineralien der Schweiz +1 other reference |
|
| 29-56. +1 other reference |
- Engiadina Bassa/Val Müstair Region
| Gröbner (2014) |
|
| Krähenbüghl H. (1982) |
|
| Stalder et al. (1998) |
|
| Soom et al. (1988) |
|
| Amir Akhavan Collection |
|
| Stalder et al. (1998) |
|
| Krähenbühl (1985) |
|
| Die Mineralien der Schweiz |
|
| Brugger J. (1996) +1 other reference |
|
| Roth (2007) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Cabalzar (1977) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Christian Bracke Collection |
|
| Stalder et al. (1998) |
|
| Cuchet et al. (2012) |
|
| Stéphane Eggs & Alexandre Salzmann |
- Albrunhorn - Turbhorn area
| |
|
| |
|
| |
|
| 50. (in German) +1 other reference |
|
| |
- Rhône gorge (Rhone gorge)
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Ansermet et al. (2012) |
|
| Ansermet et al. (2012) |
|
| EDXS analyses in May 2022 by Philippe ... |
- Mont Chemin mining district
| |
|
| |
|
| |
|
| |
|
| Stalder et al. (1998) |
|
| |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) +1 other reference |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Ansermet et al. (2012) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) +1 other reference |
|
| Stalder et al. (1998) |
|
| 86. (in German) +2 other references |
|
| Stalder et al. (1998) +1 other reference |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Stalder et al. (1998) |
|
| Ansermet et al. (2011) |
|
| Cuchet et al. (2002) |
|
| Stalder et al. (1973) |
|
| Christian Bracke Collection |
|
Syria | |
|
| Sharkov et al. (1997) |
|
Taiwan | |
|
| |
|
| Ho & Lee (1963) |
|
Tajikistan | |
|
| Turlychkin et al. (1972) |
|
| Pekov (1998) +1 other reference |
|
Tunisia | |
|
| www.archive.org (2008) |
- Sidi Jedidi (Sidi Jadidi)
| G. Brandstetter collection |
|
| Souissi et al. (Zaghouan District, North-Eastern Tunisia) |
|
Turkey | |
|
| Kupeli et al. (Farasa-Yahyali-Kayseri, Turkey) |
|
| Celebi et al. (2010) |
|
| TECHNICAL REPORT AND UPDATED RESOURCE ... |
|
| Singer et al. (2008) |
|
| Yahya Çiftçi et al. (2013) |
|
| Z. Cacsu and H. Emre (2011) |
|
| Yigit (2012) |
|
| Erik Vercammen collection and field ... +1 other reference |
|
| Kines (1969) |
|
| Yıldırım et al. (2016) |
|
| Orhan et al. (2023) |
|
| ÇAĞATAY et al. (1990) |
|
| ÇAĞATAY et al. (1990) |
|
| Beih. z. Eur. J. Mineral 18 (2006) |
|
| Demir (2009) |
|
| Akinci (2009) |
|
| Oyman et al. (2003) |
|
| Günay et al. (2022) |
|
| Erik Vercammen field observation and ... |
|
| Singer et al. (2008) |
|
| erik vercammen field observations and ... |
|
| Kumral et al. (2016) |
|
| Arik (2012) |
|
| Kuþcu et al. (2011) |
|
| Çağatay et al. (1989) |
|
| Yaylali-Abanuz et al. (2011) |
|
| Kaydu Akbudak et al. (2021) |
|
| Turkish Journal of Earth Sciences (Turkish J. Earth Sci.) |
|
Uganda | |
|
| Owor et al. (2007) |
|
UK | |
|
| Alabaster (1978) |
|
| |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| |
|
| Day (1999) |
|
| Semmons (1885) +2 other references |
|
| Collection Richard De Nul |
|
| P |
|
| Henwood (1843) +1 other reference |
|
| Henwood (1843) +1 other reference |
|
| Lapis 11 (2) |
|
| Hogg (1825) +4 other references |
|
| Hill et al. (1906) +1 other reference |
|
| Hall (1868) +1 other reference |
|
| Hill et al. (1906) |
|
| Hill et al. (1906) |
|
| Hill et al. (1906) |
|
| Phillips (1823) +3 other references |
- Clifford Amalgamated Mines
| Hall (1868) |
|
| Phillips (1823) +2 other references |
|
| Tom Hardman |
|
| Phillips (1823) +4 other references |
|
| Dines (1956) |
|
| Hill et al. (1906) |
|
| Henwood (1843) |
|
| Golley et al. (1995) |
|
| Smyth et al. (1864) |
- Perran St George and Droskyn Mines
| Henwood (1843) |
- Wheal Buller and Beauchamp
| Dana 6: 22 +4 other references |
|
| Smyth et al. (1864) +3 other references |
|
| Henwood (1843) |
|
| Le Neve Foster (1879) +2 other references |
|
| Phillips (1823) +4 other references |
|
| Phillips (1823) +1 other reference |
|
| Betterton (2000) |
|
| Henwood (1843) +2 other references |
|
| Reid et al. (1907) |
|
| Carne (1822) +1 other reference |
|
| Henwood (1843) |
|
| Golley et al. (1995) |
|
| |
|
| - (2008) |
|
| Young B. Copper mineralisation in the Permian Magnesian Limestone at Raisby et al. (2) |
|
| Bridges TF and Young B. Supergene Minerals of the Northern Pennine Orefield-A Review. in Journal of the Russell Society 7 (1) |
|
| A Thompson collection |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Thimmaiah (1956) +2 other references |
|
| Kingsbury et al. (1960) +2 other references |
|
| Hartley (1984) +2 other references |
|
| Shaw (1972) +3 other references |
|
| Mike Leppington collection (visual ID) |
|
| Cooper et al. (1990) +1 other reference |
|
| Hartley (1984) +2 other references |
|
| Day (1999) |
|
| Stanley et al. (1991) |
|
| Dunham |
|
| Day (1999) |
|
| Dunham et al. (2nd Edition) |
|
| Braithwaite (1988) +1 other reference |
|
| Day (1999) |
|
| Kingsbury (xxxx) +3 other references |
|
| Kingsbury (xxxx) +1 other reference |
|
| P. Nicholson Collection |
|
| Ex David Neal collection |
|
| M.Wirth collection |
|
| [BMS] |
|
| www.nmrs.org.uk (n.d.) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
|
| Day (1999) |
- Derbyshire Dales District
| Andrew Lawton Collection |
|
| Day (1999) |
|
| Worley (1978) |
|
| Worley (1978) |
|
| Ford et al. (1993) |
|
| Ford et al. (1993) |
|
| Ford et al. (1993) |
|
| Ford et al. (1993) |
|
| Ford et al. (1993) |
|
| Ford et al. (1993) +1 other reference |
|
| Hall (1868) +1 other reference |
|
| Brian Craik-Smith collection |
|
| Render (n.d.) |
|
| Render (n.d.) |
|
| |
|
| Render (n.d.) |
|
| Hall (1868) |
|
| P Haas collection |
|
| Day (1999) |
|
| |
|
| Hubbard et al. (2005) |
- North West Leicestershire
| BMS Collection |
|
| Day (1999) |
|
| [J.Russell Soc. 1998:41] |
|
| Day (1999) |
|
| Render (n.d.) |
|
| Dunham et al. (2nd Edition) |
|
| Malcolm Woodward collection |
|
| Dunham et al. (2nd Edition) |
|
| Dunham et al. (2nd Edition) |
|
| Dunham et al. (2nd Edition) |
|
| Render (n.d.) |
|
| Dewey et al. (1925) |
|
| |
|
| Render (n.d.) |
|
| Dewey et al. (1925) |
|
| Dewey et al. (1925) |
|
| Bill Gordon collection |
|
| Turner et al. (2010) |
|
| Burr (1996) |
|
| Render (n.d.) |
|
| |
|
| Phillips (1823) +1 other reference |
|
| Day (1999) |
|
| Smyth et al. (1864) |
- Somerset West and Taunton
| Render (n.d.) |
|
| Day (1999) |
|
| Render (n.d.) |
|
| Day (1999) |
|
| Render (n.d.) |
|
| Render (n.d.) |
|
| Render (n.d.) |
|
| |
|
| Tindle (2008) |
|
| |
|
| Moreton (2007) |
|
| Day (1999) |
|
| Moreton (2006) |
|
| |
|
| |
|
| Day (1999) |
|
| |
|
| Day (1999) |
|
| |
|
| Smyth et al. (1864) |
|
| |
|
| |
|
| S. Rust collection |
|
| |
|
| Day (1999) |
- Upper Llanfihangell-y-Creuddyn
| |
|
| |
|
| Mineralogical Magazine 1996 60 : ... +1 other reference |
|
| Day (1999) |
|
| Sutphin |
|
| Smyth et al. (1864) |
|
| Day (1999) |
|
| Day (1999) |
|
| Render (n.d.) |
|
| Day (1999) |
|
| Wolfe (2000) +1 other reference |
|
| Render (n.d.) |
|
| Render (n.d.) |
|
| S. Rust collection |
|
| Render (n.d.) |
|
| Render (n.d.) |
|
| Ian Jones collection |
|
Ukraine | |
- Goluboi Zaliv village (Limeny)
| Dvoichenko (1914) |
|
| Tischenko Alexander |
|
| Alexander I. Tischenko (1996) |
|
| w Hancewiczach na Polesiu:' Biuletyn Państwowego Instytutu Geologicznego Nr 14. Warszawa (in Polish) +4 other references |
|
| webmineral.ru (2018) |
|
| Dobrovolskaya T.I. (2004) |
|
USA | |
|
| Rocks & Min 70:5 pp 320-333 |
|
| Cook et al. (1982) |
|
| Dana 6: 1081. |
|
| Gems and Minerals of America- Jay Ellis ... |
|
| - (2008) |
|
| - (2008) |
- Denali National Park and Preserve
| USGS OFR 2004-1200 (Hawley,2004) |
|
| USGS OFR 2004-1200 (Hawley,2004) |
- Kantishna Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Fairbanks North Star Borough
- Fairbanks Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Hoonah-Angoon Census Area
| - (2008) |
- Petersburg Mining District
| - (2008) |
- Berners Bay Mining District
| mrdata.usgs.gov (n.d.) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Lake and Peninsula Borough
- Bristol Bay Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Matanuska-Susitna Borough
- Valdez Creek Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Willow Creek Mining District
| USGS OFR 98-598 (Hawley,1998) |
|
| USGS OFR 98-598 (Hawley,1998) |
|
| USGS OFR 98-598 (Hawley,1998) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Serpentine Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Chandalar Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| Econ Geol (1986) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Prince of Wales-Hyder Census Area
| - (2008) |
- Ketchikan Mining District
| - (2008) |
- South Arm of Cholmondeley Sound
| - (2008) |
|
| Copper Handbook 1911 +1 other reference |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Southeast Fairbanks Census Area
- Chistochina Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Delta River Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| USGS PP 1760A +1 other reference |
|
| - (2008) |
|
| Singer et al. (2008) |
- Valdez Creek Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Valdez-Cordova Census Area
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Chistochina Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Copper River Mining District
| - (2008) |
- Delta River Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| USGS OFR 2003-404 (Hudson,2003) +1 other reference |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Prince William Sound Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Yukon-Koyukuk Census Area
- Chandalar Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
- Hot Springs Mining District
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| - (2008) |
|
| Mason (1976) |
|
| Holden (1922) +1 other reference |
- Calumet and Arizona group of claims (Calumet and Arizona Mining Company group of claims)
| Mitchell (1920) +1 other reference |
|
| Anthony et al. (1995) |
|
| Anthony et al. (1995) |
|
| King (n.d.) |
|
| |
|
| Rob Bowell |
|
| Robert Bowell and Rolf Luetcke |
|
| Singer et al. (2005) |
|
| Dana 6:1093. +10 other references |
|
| Frondel (1949) +1 other reference |
|
| Dana 6:1093. +2 other references |
|
| |
|
| Mineralogical Record 24:421-436 |
|
| Luetcke (n.d.) |
|
| Copper Handbook 1911 |
|
| Luetcke (n.d.) |
- California Mining District (Chiricahua Mining District)
| MRDS database Dep. ID file #10258080 |
|
| Luetcke (n.d.) |
|
| Miller et al. (1983) |
|
| MRDS database Dep. ID file #10210093 |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Kellogg (1906) +1 other reference |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| MRDS database Dep. ID file #10160924 |
|
| Luetcke (n.d.) |
- Dos Cabezas Mining District (Two Heads Mining District)
| Drewes et al. (1988) |
|
| Drewes et al. (1988) |
|
| Drewes et al. (1988) |
|
| Drewes et al. (1988) |
|
| Drewes et al. (1988) |
|
| Luetcke (n.d.) |
|
| Drewes et al. (1988) |
- Middle Pass Mining District
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Anthony et al. (1995) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
- Tombstone Mining District
| Luetcke (n.d.) |
|
| Marek Chorazewicz (2023) |
|
| Butler et al. (1938b) +1 other reference |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Butler et al. (1938b) +1 other reference |
- Turquoise Mining District (Courtland-Gleeson Mining District)
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| MRDS database Dep. ID file #10048117 |
|
| - (1951, July) +1 other reference |
- Tejon group (Tejon Mining Company property)
| MRDS database Dep. ID file #10048118 |
|
| Luetcke (n.d.) |
|
| Anthony et al. (1995) |
|
| Anthony et al. (1995) |
- Yellowstone Mining District
| Luetcke (n.d.) |
|
| Bollin et al. (1958) +2 other references |
- Grandview Mining District
| Leicht (1971) +1 other reference |
- Jacob Canyon Mining District
| Fischer (1937) +1 other reference |
|
| Tainter (1947a) +1 other reference |
|
| Galbraith (1959) +1 other reference |
|
| Wenrich et al. (1992) |
|
| Anthony et al. (1995) +1 other reference |
- Prospect Canyon Mining District
| Testing at University of Arizona. |
- Vermillion Cliffs Mining District
| Scarborough (1981) |
|
| Thorne (n.d.) +2 other references |
|
| Ross (1925a) +4 other references |
|
| I visited the site on 4/18/2024 and visually identified the Cu minerals azurite et al. (as Mn dendrites) |
|
| Eastlick |
- Globe-Miami Mining District
- Globe Hills Mining District
| Sinkankas (1964) +2 other references |
|
| Dana 6: 1093. |
- Miami-Inspiration Mining District
- Castle Dome area (Castle Dome Mine area; Pinto Valley Mine area)
| Peterson (1947) +1 other reference |
- Miami-Inspiration deposit
| Reed et al. (1962) |
|
| King (n.d.) |
|
| MRDS database Dep. ID #10185792 |
- Miami-Inspiration deposit
| www.fcx.com (2019) |
|
| MRDS database Dep. ID #10184420 |
|
| MRDS database Dep. ID #10234187 |
|
| Peterson (1954) +2 other references |
|
| Galbraith (1959) +1 other reference |
|
| Peterson (1962) |
|
| Dick Thompsen collection +1 other reference |
|
| Copper Handbook 1911 |
|
| Bromfield (1956) |
|
| Lausen (1926) +1 other reference |
- Payson Mining District (Green Valley Mining District)
| Galbraith (1959) +1 other reference |
|
| Galbraith (1959) +1 other reference |
|
| Galbraith (1959) +1 other reference |
- Sunflower Mining District (Mazatzal Mountains Mining District; Quicksilver Mining District)
| Lausen (1926) +1 other reference |
|
| Lausen (1926) +1 other reference |
|
| Lausen (1926) +1 other reference |
- El Capitan Mining District
| C. Lemanski |
|
| McGoon (1962) |
- Pinal Mountains Mining District
| MRDS database Dep. ID #10209560 |
- Sierra Ancha Mining District
| Granger (1959) |
|
| Granger (1959) |
- Tonto Basin Mining District
| Anthony et al. (1995) |
|
| Simons (1964) |
|
| Simons (1964) |
|
| Simons (1964) |
|
| MRDS database MRDS ID #M050716 |
|
| Jones (1980) +1 other reference |
|
| MRDS database Dep. ID #10283143. |
|
| MRDS database Dep. ID #10258430. |
|
| Peterson et al. (1981) |
|
| Peterson et al. (1981) |
|
| Peterson et al. (1981) |
|
| Dana 6: 297 & 1094 +6 other references |
|
| - (1991) |
|
| Jim McGlasson Collection |
|
| Dana 6: 945 |
|
| Galbraith (1959) |
|
| Galbraith (1959) +1 other reference |
- Gila Hot Springs Mining District
- Chase Creek Canyon (Chase Creek Cañon)
| Dana 6:1094. |
|
| Kunz (1885) +6 other references |
|
| - (1991) |
|
| Kunz (1885) +6 other references |
|
| Kunz (1885) +5 other references |
|
| Dana 6:295 & 1094. |
|
| Anthony et al. (1995) +1 other reference |
|
| Ian Merkel specimen & photo |
|
| Keith (1978) |
|
| Fernandez (1965) +2 other references |
- Santa Maria Mining District (Planet Mining District; Swansea Mining District; Bill Williams Mining District)
| Bancroft (1911) +3 other references |
|
| Bancroft (1911) +1 other reference |
- Ellsworth Mining District
| MRDS database Dep. ID file #10186681 +1 other reference |
|
| Bancroft (1911) +2 other references |
- Imperial National Wildlife Refuge
| Pemberton (1983) |
- New River Mining District
| John Christian |
|
| MinRec 19 (3) |
|
| Lausen (1926) +1 other reference |
|
| Lausen (1926) +1 other reference |
|
| Thomas (1949) +1 other reference |
- Mineral Park Mining District
| Univ. of AZ Bull. 41 (1916-17) |
|
| Hill (1914b) +1 other reference |
|
| Hill (1914b) +1 other reference |
|
| Copper Handbook 1911 +2 other references |
- Lost Basin Mining District
| Deaderick (1980) |
|
| Theodore et al. (1987) |
|
| Reinhardt (1952) |
- Crescent channel (Channel No. 28; paleochannel No. 28)
| Witkind (1963) |
- Hunts Mesa deposit (Koley No. 2; Sam Charlie No.1; Hunt's Mesa)
| Witkind (1963) |
|
| Witkind (1963) |
- Agathla Peak (El Capitan)
| Witkind (1963) |
|
| Witkind (1963) |
|
| Witkind (1963) |
- Monument No. 1 channel (Channel No. 36)
| Witkind (1963) |
|
| Witkind (1963) |
|
| Witkind (1963) |
- Nakai Mesa Mining District
| Witkind (1963) |
|
| Witkind (1963) |
|
| Schwartz (1934) +1 other reference |
|
| . +5 other references |
- Canada del Oro Mining District (Cañada del Oro Mining District)
| Anthony et al. (1995) |
- Cerro Colorado Mining District
| Richard Dale Collection |
|
| Visual identification by Ronald Deblois |
- Cababi Mining District (Comobabi Mining District)
| Williams et al. (1963) +1 other reference |
|
| Williams et al. (1963) +1 other reference |
|
| Galbraith (1959) +1 other reference |
|
| Schrader (1915) |
|
| - (1951, July) |
|
| Wilson (1951) +2 other references |
|
| Schrader (1915) +2 other references |
|
| Schrader (1915) +5 other references |
- Greaterville Mining District
- Greaterville placer deposits
| Luetcke (n.d.) |
|
| Schrader & Hill (1915) |
- Helvetia-Rosemont Mining District
| Alexander Wong Collection (collected by Malcolm Alter) |
|
| Anthony et al. (1995) |
|
| Creasey (1955) |
|
| Luetcke (n.d.) |
|
| Schrader (1915) +1 other reference |
|
| Schrader (1915) |
|
| Rob Lavinsky |
|
| |
|
| Schrader (1915) |
|
| Betts (n.d.) |
|
| Schrader (1915) |
- Tiptop Mine group (Tiptop Camp prospects/mines)
| Schrader (1915) +2 other references |
|
| Schrader (1915) |
|
| |
|
| Schrader (1915) |
- Peach-Elgin copper deposit
| Schrader (1915) |
|
| Luetcke (n.d.) |
|
| Schrader (1915) |
- Daylight-Narragansett Mines (Narragansett-Daylight Mines)
| MRDS database Dep. ID file #10039412 |
|
| MRDS database Dep. ID file #10210685 |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Creasey (1955) |
|
| Schrader (1917) +1 other reference |
|
| Schrader (1915) |
- Robinson property (Robinson group)
| - (1915) |
|
| Granger (1962) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
- San Xavier (Tohono O'odom) Indian Reservation
- Pima Mining District (Olive Mining District; Mineral Hill Mining District; Twin Buttes Mining District)
| Self-collected and visual ID by C. ... |
- Papago Mining District (Sierrita Mining District)
| Anthony et al. (1995) |
|
| Anthony et al. (1995) |
|
| MRDS database Dep. ID file #10186590 |
|
| Personal knowledge and consultations ... |
|
| Singer et al. (2005) |
|
| personal communication |
|
| Singer et al. (2005) |
- Silver Bell Mining District
| arizona.openrepository.com (n.d.) |
|
| Copper Handbook 1911 |
|
| Engineering Mining Jour. (1904) +1 other reference |
|
| Luetcke (n.d.) |
|
| MRDS database Dep. ID file #10039906 |
|
| MRDS database Dep. ID file #10039789 |
|
| MRDS database Dep. ID file #10039766 |
|
| MRDS database Dep. ID file #10095448 |
|
| MRDS database Dep. ID file #10039817 |
|
| MRDS database Dep. ID file #10039730 |
|
| MRDS database Dep. ID file #10039768 |
|
| MRDS database Dep. ID file #10039767 |
|
| MRDS database Dep. ID file #10039765 |
|
| MRDS database Dep. ID file #10039758 |
- Indiana-Arizona Mine group (Carbonate group)
| Anthony et al. (1995) |
- Silver Hill Mining District
| Anthony et al. (1995) |
- Bunker Hill District (Copper Creek District)
- Copper Creek (Copper Creek Canyon)
| Kuhn (1951) |
|
| Kuhn (1938) +2 other references |
|
| MRDS database Dep. ID #10283179 +1 other reference |
- Florence Mining District (Blackwater Mining District; Black Rock Mining District)
| MRDS database Dep. ID #10186598 |
|
| Galbraith (1959) +1 other reference |
|
| Bideaux (1980) +1 other reference |
- Mineral Mountain Mining District
| MRDS database Dep. ID 10210499 |
|
| Wilson (1984) |
|
| MRDS database Dep. ID #10039958 |
|
| MRDS database Dep. ID #10039977 |
|
| Jones et al. (1983) |
|
| Phillips et al. (1971) +1 other reference |
|
| Phillips et al. (1971) +1 other reference |
- San Manuel Mining District
- San Manuel Mine (Apex Lead & Vanadium Mining Corp Mine; Quarelli group)
| Singer et al. (2005) |
|
| Schwartz (1949) +1 other reference |
|
| Jones (1983) |
|
| Wilson et al. (1950) |
|
| Wilson et al. (1950) +2 other references |
- Nogales Mining District (Gold Hill Mining District)
| Luetcke (n.d.) |
|
| Schrader (1915) |
- Old Baldy Mining District
| Schrader (1915) |
- Oro Blanco Mining District (Ruby Mining District)
| Schrader (1915) |
|
| Ronald Render |
- Wieland group (Wieland group claims)
| Schrader (1915) |
|
| Schrader (1915) |
- Jarilla Mine group (Jarilla Mine; Bullion Mine)
| Schrader (1915) |
|
| Schrader & Hill (1915) |
- Patagonia Mining District
| Schrader (1915) +2 other references |
|
| Schrader (1915) |
- Nash Mines group (Duquesne-Washington group)
| Luetcke (n.d.) |
|
| - (1915) +2 other references |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
- San Cayetano Mining District
| Schrader (1915) |
|
| Schrader (1917) +1 other reference |
- Glove Mine group (Zombie & Zeco claims; Festiago-Franklin; Blacksmith adit)
| Luetcke (n.d.) |
|
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) |
- Elephant Head group of claims (Tremaine-Daniels group)
| Schrader (1915) |
|
| Luetcke (n.d.) |
|
| Schrader (1915) |
|
| Schrader (1915) |
- Salero Hill (Salero Mountain)
| Schrader (1915) +2 other references |
|
| Schrader (1915) |
- Toluachi group of claims (Toluachi group; Bonanza tunnel; Tennantite claim)
| Schrader (1915) |
|
| Copper Handbook 1911 |
|
| Luetcke (n.d.) |
- Wrightson Mining District
| Schrader (1915) |
|
| Schrader (1915) |
|
| Schrader (1915) +2 other references |
|
| Schrader (1915) |
- Antelope Mountains (Antelope Range)
- Walnut Grove Mining District (Wagoner Mining District)
| MRDS database Dep. ID #10186613 |
- Black Hills (Black Hill Range)
- Black Hills Mining District
| Lindgren (1926) +3 other references |
|
| Schwartz (1938) +1 other reference |
|
| Dana 6: 1094 +1 other reference |
- Black Rock Mining District
| Luetcke (n.d.) |
- Bradshaw Mountains (Bradshaw Range)
- Agua Fria Mining District
| Collection of Barry Murphy |
|
| Brian Beck Collected |
|
| Michael Michayluk specimen |
|
| Lindgren (1926) |
- Castle Creek Mining District
| Granger (1962) |
- Hassayampa Mining District
| Lindgren (1926) |
|
| Copper Handbook 1911 |
|
| Brian Beck Collected |
|
| Copper Handbook 1911 |
- Turkey Creek Mining District
| Singer et al. (2005) |
|
| Copper Handbook 1911 |
|
| Copper Handbook 1911 |
|
| MRDS database Dep. ID #10162326 |
|
| Wilson et al. (1950) +1 other reference |
|
| Anderson et al. (1955) |
|
| Wilson et al. (1950) +1 other reference |
|
| Anderson et al. (1955) |
|
| Copper Handbook 1911 |
|
| Johnston (1961) +1 other reference |
|
| MRDS database Dep. ID #10259519 |
- Fortuna Mining District (La Fortuna Mining District)
- Barry M. Goldwater Air Force Range
| Wilson (1933) +1 other reference |
- Muggins Mountains uranium prospects
| Outerbridge et al. (1960) +1 other reference |
|
| Smith (1988) |
|
| Howard (1987) +1 other reference |
|
| Howard (1987) +1 other reference |
|
| Howard (1987) +1 other reference |
|
| Rocks & Min.: 63:122 +1 other reference |
|
| Howard (1987) +1 other reference |
|
| Rocks & Min.:63:123 +1 other reference |
|
| Rocks & Min.: 63:123. |
|
| Rocks & Min.:63:123 +1 other reference |
|
| Van Nostrand Reinholt Press: 224. +2 other references |
|
| - (2005) |
- Murphys Mining District (Esmeralda Mining District; Sperry Mining District)
| - (2005) |
- Campo Seco Mining District
| - (2005) |
|
| [… Geological Society of America ... +7 other references |
|
| being a report of the California State ... +4 other references |
|
| - (2005) |
|
| Van Nostrand Reinholt Press: 224. USGS ... +4 other references |
- Pilot Hill Mining District
| Jay Buscio |
- Rattlesnake Bar Mining District
| Van Nostrand Reinholt Press: 224. +4 other references |
- Big Creek-Rush Creek Mining District
| Walstrom (n.d.) |
- Cargo Muchacho Mining District (Hedges Mining District; Ogilby Mining District)
| Pemberton (1983) |
|
| Van Nostrand Reinholt Press: 224. +2 other references |
- Paymaster Mining District
| - (2005) |
|
| Van Nostrand Reinholt Press: 224. +4 other references |
|
| Van Nostrand Reinholt Press: 224. +4 other references |
|
| Van Nostrand Reinholt Press: 224. +2 other references |
|
| Todd et al. (1987) |
- Lookout Mining District (Modoc Mining District)
| Hanks (1884) +1 other reference |
- Zinc Hill Mining District
| USGS PP 368 |
|
| Van Nostrand Reinholt Press: 225 +3 other references |
|
| Darwin quadrangle +4 other references |
- Darwin Mine (Anaconda; Bernon Mine; Defiance Mine; Essex Mine; Independence Mine; Intermediate Mine; Rip Van Winkle Mine; Thompson Mine)
| Rocks & Min.: 23:504-507. |
|
| Hall (1963) |
|
| Waring (1919) +1 other reference |
|
| USGS Bull 540 |
|
| Calif. Div. of Mines & Geology Bull. #189 et al. (1966) +1 other reference |
|
| 15th Report of the State Mineralogist +8 other references |
|
| USGS Bull 540 |
|
| Van Nostrand Reinholt Press: 225. +1 other reference |
|
| - (2005) |
|
| In the collection of Brent Thorne. ... +1 other reference |
|
| Rocks & Min.: 14:72. +1 other reference |
|
| B.A.M.-bay area mineralogists |
- Panamint Mts (Panamint Range)
| Van Nostrand Reinholt Press: 225. +2 other references |
|
| Copper Handbook 1911 |
|
| Murphy (1930) +1 other reference |
- Wildrose Mining District (Wild Rose Mining District)
| Slaughter (2023) |
|
| USGS Bull 540 |
|
| Van Nostrand Reinholt Press: 225. +3 other references |
- East Mayacmas Mining District
| - (1908, September) +1 other reference |
|
| Van Nostrand Reinholt Press: 225. +2 other references |
|
| Van Nostrand Reinholt Press: 225. +3 other references |
|
| Catalog: Mineral Sciences Collection |
|
| Mining and Scientific Press (1864) +1 other reference |
|
| Van Nostrand Reinholt Press: 225. +2 other references |
|
| - (2005) |
|
| Van Nostrand Reinholt Press: 225. +3 other references |
|
| Van Nostrand Reinholt Press: 225. +3 other references |
|
| Pelletier |
- Green Mountain Mining District (Ben Hur Mining District)
| Van Nostrand Reinholt Press: 225. +3 other references |
|
| Van Nostrand Reinholt Press: 225. +2 other references |
|
| Van Nostrand Reinholt Press: 225. +3 other references |
|
| Murdoch et al. (1966) |
- Cathey Mining District (Cathay Mining District)
| Copper Handbook 1911 |
- Hunter Valley Mining District
| Dana 6: 1096 +3 other references |
|
| Aubury (1905) +1 other reference |
- Red Mountain Mining District
| Erd et al. (1981) |
|
| Pabst (1977) |
|
| Tucker (1919) +1 other reference |
|
| Ross (1941) |
|
| Van Nostrand Reinholt Press: 225-226. +2 other references |
|
| King (n.d.) |
|
| Emmons et al. (1885) +2 other references |
|
| Ransome (1894) +1 other reference |
|
| Emmons et al. (1885) |
|
| Rinehart (1956) |
|
| Rinehart (1956) |
|
| Whiting (1888) +1 other reference |
|
| Ransome (1940) +1 other reference |
|
| Van Nostrand Reinholt Press: 226. +4 other references |
|
| Lindgren (1896a) |
|
| - (1908, September) +1 other reference |
|
| Van Nostrand Reinholt Press: 226. +3 other references |
- Wheatland Mining District
| Silliman et al. (1867a) +1 other reference |
|
| Copper Handbook 1911 |
- Genesee Valley Mining District (Genesee Mining District)
| Diller (1905) |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| - (2005) |
|
| Diller (1905) |
- Taylorsville Mining District
| Singer et al. (2008) |
|
| www.mineralsocal.org |
|
| USGS Bulletin 260 p47 |
|
| CDMG OFR 94-11 " Mineral Land ... |
|
| Orcutt (1890) +1 other reference |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
- Eagle Mountains Mining District (Monte Negro Mining District)
| Tucker (1924a) +1 other reference |
|
| CDMG OFR 94-11 " Mineral Land ... |
|
| Woodford et al. (1941) |
|
| Woodford et al. (1941) |
|
| Van Nostrand Reinholt Press: 226 +3 other references |
- Arlington District (Ironwood District)
| Merrill (1919) +1 other reference |
|
| Copper Handbook 1911 |
|
| Merrill (1919) +1 other reference |
- Mule Mountains District (Hodges Mountain District)
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| - (2005) |
|
| California Division of Mines and Geology Open-File Report 94-11 (1994) |
|
| Tucker et al. (1929) |
- Riverside Mts (Siakariri)
- Bendigo District (Riverside Mountain District)
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| CDMG OFR 88-20 "Mineral Land ... |
|
| CDMG OFR 88-20 "Mineral Land ... |
|
| CDMG OFR 88-20 "Mineral Land ... |
|
| CDMG OFR 88-20 "Mineral Land ... |
- Arrowhead District (Arrow District)
| - (2005) |
|
| MarekC pers. coll. 2019 |
- Belleville Mining District
| Tucker et al. (1943) +3 other references |
- Rodman Mountains high point
| Tucker et al. (1943) +2 other references |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 227. +4 other references |
- Bullion Mts (Bullion Range)
- Stedman District (Ludlow; Rochester; Buckeye)
| - (2005) |
- Calico Mts (Calico Hills)
| Weeks (1925) +2 other references |
|
| Cloudman et al. (1919) +1 other reference |
- Clark Mts (Clark Mountain Range)
- Clark Mountain District (Clark District)
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Kepper et al. (2000) |
|
| Van Nostrand Reinholt Press: 108. +2 other references |
|
| Van Nostrand Reinholt Press: 227 +2 other references |
|
| Berger et al. (2009) |
- Halloran Springs District
| 27th Report of the State Mineralogist +5 other references |
|
| Van Nostrand Reinholt Press: 226. +4 other references |
|
| Ronald Wynn Sheets et al. (1995) |
|
| Van Nostrand Reinholt Press: 226. +4 other references |
- Kokoweef Peak (Kokoweep Peak; Koko Weef Mountain)
| Van Nostrand Reinholt Press: 226. +4 other references |
|
| 27th Report of the State Mineralogist +6 other references |
|
| Cloudman et al. (1919) +1 other reference |
|
| Tucker (1940a) +2 other references |
|
| Weber (1963) +2 other references |
|
| Weber (1963) |
|
| R. Housley |
|
| Tucker (1921) +1 other reference |
|
| Cloudman et al. (1919) +1 other reference |
- Signal Mining District (Vontrigger Mining District)
| Van Nostrand Reinholt Press: 227. +4 other references |
- Silver Mountain Mining District
| Van Nostrand Reinholt Press: 226-227. +4 other references |
- Whipple District (Monumental District)
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 226. +2 other references |
|
| Van Nostrand Reinholt Press: 227. +4 other references |
|
| Cloudman et al. (1919) +1 other reference |
|
| Huguenin (1921) +1 other reference |
- New Almaden Mining District
| Kunz (1905) +1 other reference |
|
| Van Nostrand Reinholt Press: 91 +2 other references |
|
| Murdoch (1966) |
- East Shasta Copper - Zinc Mining District
- Bully Hill District (Winthrop District)
| Diller (1903) +1 other reference |
|
| Van Nostrand Reinholt Press: 228. +2 other references |
|
| Van Nostrand Reinholt Press: 228. +2 other references |
- French Gulch Mining District
| Van Nostrand Reinholt Press: 32. +3 other references |
- West Shasta Copper - Zinc Mining District
| Van Nostrand Reinholt Press: 228. +2 other references |
|
| Logan (1925) +1 other reference |
|
| Murdoch (1966) |
- Callahan Mining District (Scott River Mining District)
| Van Nostrand Reinholt Press: 228. +2 other references |
|
| Murdoch et al. (1966) |
- Guerneville Mining District
| - (1908, September) +1 other reference |
- West Mayacmas Mining District
| Bradley (1916a) +1 other reference |
|
| Van Nostrand Reinholt Press: 228. +4 other references |
- New River Mining District
| Van Nostrand Reinholt Press: 228. +3 other references |
- South Fork Mining District
| Van Nostrand Reinholt Press: 228. +3 other references |
|
| - (2005) |
|
| Van Nostrand Reinholt Press: 228. +2 other references |
|
| Franke (1930) +1 other reference |
|
| Van Nostrand Reinholt Press: 228. +2 other references |
|
| Franke (1930) +1 other reference |
- Chinese Camp Mining District
| Van Nostrand Reinholt Press: 228. +3 other references |
- Coulterville Mining District
| Roberts et al. (1994) +1 other reference |
- Jamestown Mining District
| Van Nostrand Reinholt Press: 228. +4 other references |
|
| DeVito (1991) |
|
| Van Nostrand Reinholt Press: 183. +2 other references |
|
| Murdoch et al. (1966) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
- Jamestown Mining District
| Eckel et al. (1997) |
- Sugarloaf Mining District
| Emmons et al. (1885) |
|
| Eckel et al. (1997) |
- Chalk Cliff Mining District
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Eckel et al. (1997) |
|
| Dings et al. (1957) +1 other reference |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| U.S. Geological Survey Professional ... +1 other reference |
- Freeland-Lamartine Mining District
| Rocks & Min.:65:58 +1 other reference |
|
| USGS PP 223 (Lovering,1950) |
|
| Lovering et al. (1950) |
- Idaho Springs Mining District (Virginia Mining District)
| USGS OFR 66-87 (Moench,1966) |
|
| Emmons et al. (1885) |
- Lamartine Mining District
| Dana 6:1089. |
|
| Dana 6:1089. |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
- Lawson Mining District (Dumont Mining District; Montana Mining District)
| Moench et al. (1966) |
- Upper Union Mining District (Empire Mining District)
| USGS PP 223 (Lovering,1950) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
- Mount Wilson Mining District
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Engineering and Mining Journal (1913) |
|
| Eckel et al. (1997) |
|
| Lovering et al. (1950) +1 other reference |
|
| Am Min 50:1179-1212 |
|
| C.R. Carnein collection +1 other reference |
- Eight Mile Park Pegmatite Mining District
| Eckel et al. (1997) |
|
| Pearl |
|
| Eckel et al. (1997) |
- Black Hawk Mining District
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Sims (1963) |
- Dorchester Mining District
| Eckel et al. (1997) |
- Elk Mountain Mining District (Snowmass Mountain Mining District)
| Eckel et al. (1997) |
- Gold Brick Mining District
| Eckel et al. (1997) |
- Quartz Creek Mining District
| Dings et al. (1957) |
- Spring Creek (Spring Gulch)
| Eckel et al. (1997) |
- Tincup Mining District (Pieplant Mining District)
| Dings et al. (1957) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) |
- Tomichi Mining District (White Pine Mining District)
| Eckel et al. (1997) |
|
| Dings et al. (1957) |
|
| Dings et al. (1957) +1 other reference |
- Galena Mining District (Henson Creek Mining District)
| Rocks & Min.: 65:59. |
|
| Eckel et al. (1997) |
|
| Minerals of Colorado (1997) |
- Clear Creek pegmatite Province
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
- Evergreen Mining District (Malachite Mining District)
- Bear Creek-Evergreen area
| Eckel et al. (1997) |
- Ralston Buttes Mining District
| USGS: Geological Survey Circular 320 |
|
| Page et al. (1956) +2 other references |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Summerlin (2014) |
|
| Summerlin (2014) |
|
| USGS Bull 1434 |
|
| Geology and Mining Industry of Leadville |
|
| Frank Karasti |
|
| James A. Bauer +1 other reference |
|
| Mineralogical Record:16 (3) |
- Empire Mining District (Howes Gulch Mining District)
| Eckel et al. (1997) |
- Prairie Divide Mining District (St. Cloud Mining District)
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Rocks & Min 70:5 pp 298-310 |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Personally collected by Dennis & Tammey ... |
|
| Eckel et al. (1997) |
|
| Beroni +2 other references |
|
| Patrick Haynes. Minerals not verified ... |
- La Sal Mining District (Paradox Valley Mining District)
| Eckel et al. (1997) |
|
| self-collected on 9/4/2010 |
|
| Econ Geol (1986) |
|
| Bullock (1981) |
|
| Dana Slaughter Minerals dealer ... |
|
| Rocks & Min 70:5 pp 298-310 +1 other reference |
|
| Rocks & Minerals 82:368-380 +1 other reference |
|
| U.S. Geological Survey Professional ... +1 other reference |
|
| Rocks & Min.: 23:704. +3 other references |
|
| Rocks & Minerals 82:368-380 |
|
| Singewald +1 other reference |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| 2008 New Mexico Mineral Symposium ... +2 other references |
- Horseshoe Basin Mining District
| Rocks & Minerals 81:356-361 |
|
| Singewald +1 other reference |
|
| Eckel et al. (1997) |
|
| Eckel et al. (1997) |
|
| Lovering et al. (1950) |
|
| Eckel et al. (1997) |
|
| Emmons et al. (1885) |
|
| Eckel et al. (1997) |
|
| Econ Geol (1991) |
|
| Rocks & Min.:63:275-277 & 65:59. +1 other reference |
- Lower San Miguel Mining District
- Placerville Mining District
| Eckel et al. (1997) |
|
| Wilmarth |
- Slick Rock Mining District
| Shawe et al. (2011) |
|
| Thorne (n.d.) |
|
| In the collection of Alex Earl |
- Argentine Mining District
| Lovering et al. (1950) |
|
| Erich Laskowski Collection--Personally ... |
|
| Lovering et al. (1950) |
|
| USGS PP 75 (Ransome,1911) |
|
| Eckel et al. (1997) |
|
| USGS PP 223 (Lovering,1950) |
|
| Henderson et al. (1967) |
|
| J. Zolan Collec. |
|
| Wilson (2001) |
|
| Allan Young. +1 other reference |
|
| Longwell (1932) |
|
| personal identification |
|
| Januzzi et al. (1976) |
|
| personal identification by Fred Schuster |
|
| Shepard (1837) |
|
| LeTourneau (1985) |
|
| Januzzi et al. (1976) |
|
| woodburylibraryct.org (2020) |
|
| Schooner (circa 1985) |
|
| Januzzi et al. (1976) +1 other reference |
|
| Shepard |
|
| Jeremy Zolan collection |
- Cartersville Mining District
| Cook (1978) |
|
| Rocks & Min.: 64:196. |
|
| Rocks & Min.:64:198. |
|
| Rocks & Min.:64:198. |
- Dill Mine (Paschal Mine; Julia Mine; Phelps Mine)
| Rocks & Min.: 64:200. |
|
| Rocks & Min.: 64: 198 & 422. |
|
| Rocks & Min.: 64:201. |
|
| Gems and Minerals of America- Jay ... |
|
| Cook (1978) |
|
| Rocks & Min. 70:244 |
|
| Ream (2004) |
|
| Copper Handbook 1911 +1 other reference |
|
| Betts (n.d.) |
|
| USGS Bull 470 |
- St. Charles Mining District
| USGS Bull 470 +1 other reference |
|
| USGS Bull 2064X |
- Lava Creek Mining District
| Ream (1995) |
|
| Ream (1995) |
- Skull Canyon Mining District
| Ream (1995) |
|
| Rocks & Min. 70:249-250. |
|
| Ream (2004) |
|
| Ream (2004) |
|
| Ream (1995) |
|
| Ream (2004) |
|
| Ream (2004) |
- Frank Church–River of No Return Wilderness
| Ream (2004) |
|
| Ream (2004) |
|
| Rocks & Minerals (1995) |
|
| Koschmann et al. (1968) |
|
| Ream (2004) |
|
| Ream (2004) |
|
| Ream (2004) |
- Yankee Fork Mining District
| Ream (1995) |
- Gold Hill Mining District (Blackfoot Mining District)
| Ream (1995) |
|
| Ream (1995) |
- Blue Wing Mining District
| Ream (1995) |
- Eldorado Mining District (Geertson Mining District)
| Ream (1995) |
|
| Ream (1995) |
|
| Ream (1995) |
|
| Ream (2004) |
- Gilmore Mining District (Texas Mining District)
| USGS PP 1480 (Ruppel,1988) |
|
| Ream (1995) |
- Junction Mining District (Little Eightmile Mining District)
| Ream (2004) |
|
| Ream (2004) |
|
| Ream (1995) |
|
| Ream (1995) |
|
| Ream (1995) |
|
| Ream (1995) |
|
| Ream (2004) |
|
| Ream (1995) |
- Sandy Creek Mining District
| Ream (2004) |
- Spring Mountain Mining District
| Ream (2004) |
|
| Ream (2004) |
- Silver City Mining District (Carson Mining District)
| Collins (2012) |
- War Eagle Mine (South Chariot; Orofino; Golden Chariot; Mahogany; Sinker; Ida Elmore)
| Collins (2012) |
|
| Rocks & Min. 70:255 |
|
| Ream (2004) |
|
| Mineral Record (1981) |
|
| Dana 7:I:493. +1 other reference |
- Evolution Mining District
| MRDS 10241626 |
|
| Copper Handbook 1911 |
|
| Ream (1995) +1 other reference |
|
| Rocks & Min. 70:262 +1 other reference |
- Little North Fork Mining District
| Umpleby et al. (1923) |
- Placer Center Mining District
| Ream (2004) |
|
| Umpleby et al. (1923) |
- St. Regis Mining District
| Ream (2004) |
|
| Ream (2004) |
- Cave-In-Rock Mining Sub-District
| Mineralogical Record |
|
| Mineralogical Record (1997) |
|
| Rocks & Minerals: 63: 359. +1 other reference |
- Spar Mountain (Iron Mountain)
| Denny et al. (2020) |
|
| Mineralogical Record (1997) |
|
| Mineralogical Record (1997) |
|
| Smith et al. (2002) |
|
| Economic Geology (1990) |
|
| Economic Geology (1990) |
|
| R&M 69:3 pp 156-162 |
- Blue Hill - Castine Mining District
| Dana 6:1054 only specifies a generic ... |
|
| King et al. (1994) |
|
| King et al. (1994) |
|
| - (2005) |
|
| King (n.d.) |
|
| King et al. (1994) |
|
| King et al. (1994) |
|
| King et al. (1994) |
|
| Rocks & Min.: 21:144-145 +1 other reference |
|
| Heyl et al. (1965) |
|
| Rocks & Min.: 22:1023. |
|
| Heyl et al. (1965) |
|
| Ostrander (1940) |
|
| Mines of the Washington D.C Area |
|
| Rocks & Min.: 22:1023. |
|
| Rocks & Min.: 21:665. |
|
| Heyl et al. (1965) |
|
| Ref: Minerals of the Washington |
|
| Bernstein (1980) |
|
| Cordua (1969) |
|
| Gleba (2008) |
|
| Shockley (1874) |
|
| Emerson (1895) |
|
| Former Alfred Patrie collection |
|
| |
|
| Rocks & Minerals: 23: 594-597. |
|
| Harvard Museum of Natural History |
|
| P Cristofono collection 2010 |
|
| Gleba (2008) |
|
| Harvard Museum of Natural History |
|
| Gleba (2008) |
|
| Carlson et al. (2007) |
|
| Heinrich et al. (2004) |
|
| Dana's System of Mineralogy 6th ed. |
|
| Heinrich et al. (2004) |
|
| Morris Jr. (1983) |
|
| Yalmer Primeau collection |
|
| Heinrich et al. (2004) |
|
| Yalmer Primeau collection |
|
| Heinrich et al. (2004) |
|
| Bornhorst |
|
| Dana 6: 1086. |
|
| Dana 6: 1086. |
|
| Rosemeyer (1996) |
|
| Heinrich et al. (2004) |
|
| Heinrich et al. (2004) |
|
| Heinrich et al. (2004) |
|
| Rosemeyer et al. (2000) |
- Ishpeming Greenstone belt (Marquette Greenstone belt)
| Heinrich et al. (2004) |
|
| Heinrich et al. (2004) |
|
| Field collected by Dan Fountain ca. 2000 |
|
| Carlson et al. (2004) |
|
| Heinrich et al. (2004) |
|
| Frank Karasti |
|
| Rocks & Min.: 18:302-303. |
|
| Eby et al. (1897) |
|
| Garland C Broadhead (1874) |
|
| Dana 6:1083. |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Dana 6: 1083. |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Sherwood et al. (1998) |
|
| Nicholson (1882) |
|
| Dana 6:1084. |
|
| Gobla (2012) |
|
| Koschmann et al. (1968) |
|
| Winchell (1914) |
|
| Winchell (1914) |
- Beaverhead Mining District (Dark Horse Mining District)
| Winchell (1914) |
|
| Winchell (1914) |
|
| Gobla (2012) |
|
| Gobla (2012) |
|
| Winchell (1914) +1 other reference |
|
| Karlstrom |
|
| Gobla (2012) |
|
| Thorne (n.d.) |
|
| Gobla (2012) |
|
| Gabe Cangelosi Collection |
|
| Gabe Cangelosi Collection |
- Elkhorn Mining District (Coolidge Mining District; Wise River Mining District)
| Winchell (1914) |
|
| Winchell (1914) |
|
| Personally collected |
- Rock Creek Mining District
| Myers (1952) |
- Utopia Mining District (Birch Creek Mining District)
| Ref: Montana Bureau of Mines and Gology ... +2 other references |
- Park Mining District (Indian Creek Mining District)
| Ore Deposits of the Northern part of the Park (Indian Creek) +2 other references |
- Georgetown Mining District (Southern Cross Mining District; Cable Mining District)
| Mines and Mineral Deposits of the ... +1 other reference |
|
| Mines and Mineral Deposits of the ... +1 other reference |
- Johnson Basin Mining District
| Gobla (2012) |
- Lost Creek Mining District
| Mines and Mineral Deposits of the ... +1 other reference |
- Silver Lake Mining District
| Mines and Mineral Deposits of the ... +1 other reference |
- Warm Springs District (Gilt Edge District; Gold Hill District; Maiden District)
| Gobla (2012) |
|
| Gobla (2012) |
- Philipsburg Mining District (Flint Creek Mining District)
| Waisman (1992) +1 other reference |
|
| Geology and Ore Deposits of the ... +1 other reference |
|
| Min News 21:7 p 1 |
- South Boulder Creek Mining District (Princeton Mining District)
| Mines and Mineral Deposits of the ... +1 other reference |
|
| Min News 20:6 pp11-12 |
|
| Rocks & Minerals 83:1 pp 20-33 |
|
| Igneous Rocks and Related Mineral ... +1 other reference |
|
| Chris Tucker and John Betts specimens. +1 other reference |
|
| Gabe Cangelosi Collection. +1 other reference |
- Wolf Creek Mining District
| Chris Tucker and John Betts specimens. +1 other reference |
- Libby Mining District (Snowshoe Mining District)
| Gobla (2012) |
- Mineral Hill Mining District (Pony Mining District)
| MBMG IC-24 (Reid,1958) |
|
| Winchell (1914) |
- South Boulder Mining District
| Winchell (1914) |
- Tidal Wave Mining District
| Winchell (1914) |
|
| Winchell (1914) |
|
| USGS OFR 1945-34 |
- Musselshell Mining District (Copperopolis Mining District)
| Gobla (2012) |
|
| USGS Bull 139 |
- Copper Cliff Mining District
| Gobla (2012) |
|
| Copper Handbook 1911 |
- Thompson River Mining District
| Gobla (2012) |
- Butte Mining District (Summit Valley Mining District)
| Jenkins et al. (2002) |
|
| Mineralogical Record 33:1 |
|
| Weed (1912) |
|
| Winchell (1914) |
- Divide Creek Mining District
| Winchell (1914) |
|
| Winchell (1914) |
|
| Winchell (1914) |
|
| Winchell (1914) |
- Copper Kettle Mining District
| USBM Inf Circ 7093 (1939) |
|
| Castor et al. (2004) |
- Holy Cross Mining District
| Castor et al. (2004) |
|
| J.V. Tingley and J. Quade (1987) |
- Table Mountain Mining District
| USBM Inf Circ 7093 (1939) |
|
| NBMG Bull 83 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| Harlan (1984) |
- Gold Butte Mining District
| Longwell et al. (1965) |
|
| Longwell et al. (1965) |
|
| Longwell et al. (1965) |
|
| Longwell et al. (1965) |
|
| Singer et al. (2005) |
|
| Longwell et al. (1965) |
- Searchlight Mining District
| Longwell et al. (1965) |
|
| Longwell et al. (1965) |
|
| Castor et al. (2004) |
- Goodsprings Mining District
| Longwell et al. (1965) |
|
| Longwell et al. (1965) |
|
| USGS PP 162] +1 other reference |
|
| Longwell et al. (1965) |
|
| USGS PP 162] +1 other reference |
|
| USGS PP 162] +1 other reference |
|
| Longwell et al. (1965) |
|
| USGS PP 162] +1 other reference |
|
| Longwell et al. (1965) |
|
| USGS Bul 540 |
|
| Longwell et al. (1965) |
|
| Castor et al. (2004) |
- Bunkerville Mining District
| Schilling (1979) +1 other reference |
|
| Schilling (1979) |
|
| LaPointe et al. (199) +1 other reference |
|
| LaPointe et al. (199) |
|
| Castor et al. (2004) |
|
| LaPointe et al. (199) |
|
| Castor et al. (2004) |
|
| LaPointe et al. (199) +1 other reference |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
- Corral Creek Mining District
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
- Dolly Varden Mining District
| Castor et al. (2004) +2 other references |
|
| Castor et al. (2004) |
- Elk Mountain Mining District
| LaPointe et al. (199) |
|
| Castor et al. (2004) |
|
| USGS Bull 741 |
|
| USGS Open-File Report 76-56 |
- Lafayette Mining District
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| LaPointe et al. (199) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| LaPointe et al. (199) |
|
| - (2005) |
|
| LaPointe et al. (199) |
- Mountain City Mining District
| USGS Open-File Report 76-56 +2 other references |
|
| USGS Bull 1162B |
|
| USGS Open-File Report 76-56 +1 other reference |
|
| LaPointe et al. (199) |
|
| - (2005) |
|
| LaPointe et al. (199) |
- Spruce Mountain Mining District
| Castor et al. (2004) |
- Crow Springs Mining District
| Schilling (1979) |
|
| NBMG Bull 78 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| NBMG Bull 78 Geology and Mineral ... |
- Gold Point Mining District
| Schilling (1979) +1 other reference |
|
| Castor et al. (2004) |
|
| Copper Handbook 1911 |
|
| Sydney Hobart Ball (1907) |
- Lone Mountain Mining District
| Castor et al. (2004) |
|
| - (2005) |
|
| NBMG Bull 78 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| - (2005) |
- Maggie Creek Mining Subdistrict
| Jensen et al. (1995) |
|
| NBMG Bull 64 Geology and Mineral ... |
|
| NBMG Bull 64 Geology and Mineral ... |
|
| - (2005) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
- Lone Mountain Mining District
| NBMG Bull 64 Geology and Mineral ... |
|
| Mineralogical Record 26:467 |
|
| NBMG Bull 64 Geology and Mineral ... |
- Maggie Creek Mining District
| John W. Norby and Michael J.T. Orobona (2002) |
|
| NBMG Bull 64 Geology and Mineral ... +1 other reference |
|
| NBMG Bull 64 Geology and Mineral ... +1 other reference |
- Mineral Hill Mining District
| NBMG Bull 64 Geology and Mineral ... |
- Modarelli-Frenchie Creek Mining District
| Castor et al. (2004) |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Castor et al. (2004) |
- Battle Mountain Mining District
| NBMG Bull 59 Geology and Mineral ... |
- Iron Point Mining District
| Castor et al. (2004) |
- Jackson Mountains Mining District
| Castor et al. (2004) |
- Leonard Creek Mining District
| Schilling (1979) +1 other reference |
|
| Bowell et al. (2005) |
- Red Butte Mining District
| NBMG Bull 59 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| - (2005) |
- Varyville Mining District
| NBMG Bull 59 Geology and Mineral ... |
- Battle Mountain Mining District
| USGS Prof Paper 798D +1 other reference |
|
| USGS Prof Paper 798D |
|
| Castor et al. (2004) +1 other reference |
|
| - (2005) |
|
| Schilling (1979) +1 other reference |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Erich Laskowski Collection +1 other reference |
- Big Creek Mining District
| Castor et al. (2004) |
|
| - (2005) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Ranta (1967) +1 other reference |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Koschmann et al. (1968) |
- North Battle Mountain Mining District
| Stewart et al. (1977) |
|
| Stewart et al. (1977) |
|
| Castor et al. (2004) |
|
| - (2005) |
|
| Castor et al. (2004) |
|
| - (2005) |
|
| Castor et al. (2004) |
- Little Mountain Mining District
| - (2005) |
- Pennsylvania Mining District
| - (2005) |
|
| Castor et al. (2004) |
|
| NBMG Bull 73 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
- Tem Piute Mining District
| - (2005) |
|
| - (2005) |
- Yerington Mining District
| NBMG Bull 73 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| Doebrich +3 other references |
|
| NBMG Bull 73 Geology and Mineral ... |
|
| Pullman |
|
| NBMG Bull 73 Geology and Mineral ... |
|
| - (2005) |
|
| Castor et al. (2004) |
- Candelaria Mining District
| Collection of Kelly Starnes 1990 |
|
| Ross (1961) |
|
| - (2005) |
|
| Ross (1961) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| - (2005) |
|
| USBM Inf Circ 6941 (1938) |
|
| Ross (1961) |
|
| Singer et al. (2005) |
|
| Ross (1961) +1 other reference |
|
| Ross (1961) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Doebrich +2 other references |
|
| Castor et al. (2004) |
|
| Ross (1961) |
- Mountain View Mining District
| Castor et al. (2004) |
|
| Ross (1961) |
|
| U.S.G.S. Bulletin 973E |
|
| Ross (1961) |
|
| Schilling (1979) |
|
| Ross (1961) |
|
| U.S.G.S. Bulletin 973E |
|
| Castor et al. (2004) |
|
| Rocks & Min.: 21:200-203. |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Singer et al. (2005) |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| - (2005) |
|
| Castor et al. (2004) |
|
| Rocks & Minerals (2010) |
- Ellendale Mining District
| NBMG Bull 99B Geology and Mineral ... |
|
| NBMG Bull 99B Geology and Mineral ... |
- Queen City Mining District
| NBMG Bull 77 Geology and Mineral ... |
- Willow Creek Mining District
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| NBMG Bull 99B Geology and Mineral ... +1 other reference |
|
| NBMG Bull 99B Geology and Mineral ... +2 other references |
- San Antone Mining District
| NBMG Bull 99B Geology and Mineral ... |
|
| Schilling (1979) +1 other reference |
|
| Castor et al. (2004) |
|
| NBMG Bull 77 Geology and Mineral ... |
- Cloverdale Mining District
| NBMG Bull 99B Geology and Mineral ... |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| NBMG Bull 99B Geology and Mineral ... |
- Twin River Mining District
| NBMG Bull 99B Geology and Mineral ... |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Castor et al. (2004) |
- Jefferson Canyon Mining District
| NBMG Bull 99B Geology and Mineral ... +1 other reference |
- Manhattan Mining District
| Castor et al. (2004) +1 other reference |
- Northumberland Mining District
| MinRec 16 (1) |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Erich Laskowski Collection |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| NBMG Bull 99B Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Ameican Mineralogist +2 other references |
|
| Luetcke (n.d.) |
|
| Luetcke (n.d.) |
|
| Johnson (1977) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| Johnson (1977) |
|
| Johnson (1977) |
|
| - (2005) |
- Juniper Range Mining District
| Johnson (1977) |
- Muttlebury Mining District
| Johnson (1977) |
|
| Johnson (1977) |
|
| - (2005) |
|
| Johnson (1977) |
- Stateline Peak Mining District
| NBMG Bull 70 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| NBMG Bull 70 Geology and Mineral ... |
|
| NBMG Bull 70 Geology and Mineral ... |
- Pah Rah Range (Pah Rah Mts)
- Olinghouse Mining District (White Horse Mining District)
| R&M 79:1 p44-54 |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| NBMG Bull 70 Geology and Mineral ... |
|
| Castor et al. (2004) |
|
| NBMG Bull 70 Geology and Mineral ... |
|
| Castor et al. (2004) |
- Bald Mountain Mining District
| Castor et al. (2004) |
|
| - (1976) |
|
| - (2005) |
- Cherry Creek Mining District
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (1976) |
|
| - (2005) |
- Huntington Creek Mining District
| - (1976) |
|
| - (1976) |
- Lexington Mining District
| - (2005) |
- Muncy Creek Mining District
| - (1976) +1 other reference |
|
| - (1976) |
|
| Rocks & Minerals. Nov. 1999. |
|
| Castor et al. (2004) |
|
| Castor et al. (2004) |
|
| - (1976) |
|
| - (2005) |
|
| - (1976) |
- Schellbourne Mining District
| - (2005) |
- Silver Canyon Mining District
| - (1976) |
|
| Castor et al. (2004) |
- White Pine Mining District
| - (1976) |
|
| John Chipman collection |
|
| Morrill |
|
| Smith (2005) |
|
| |
|
| Whitmore et al. (2004) |
|
| Meyers et al. (1956) |
|
| Rocks & Min.: 17:136-139. +1 other reference |
|
| Nashua Min Soc. Disp. Cat. 1995 |
|
| New Jersey State Geologist's Annual Report (1906) +1 other reference |
|
| The Minerals of NYC & Its Environs et al. (1931) |
|
| Minerals of Laurel Hill et al. (published privately) |
|
| Rocks & Min.: 21:346-347. +1 other reference |
|
| NJ State Geol. Ann. Rpt. (1888) |
|
| Rocks & Min.: 20:207-209. +1 other reference |
|
| NJ State Geol. Ann. Rpt. (1906) |
|
| Manchester (1931) |
|
| Rocks & Min.: 19:306-309. |
|
| Manchester (1931) |
|
| Rocks & Min.: 59:15 +1 other reference |
|
| A Quest for New Jersey Minerals (1978) |
|
| Ostrander et al. (1940) |
|
| The Iron Mines of NJ (1910) |
|
| Betancourt (The Picking Table, Vol. 27) |
|
| Palache (1935) +1 other reference |
|
| FOMS Millsite Committee (1986) |
|
| Manchester (1931) |
|
| King (n.d.) |
|
| Earl Verbeek +1 other reference |
|
| Palache (1935) +1 other reference |
|
| Observed 10/2009 by David S. Bernstein |
|
| David S. Bernstein +2 other references |
|
| NJ State Geol. Ann. Rpt. (1900) +1 other reference |
|
| Patrick Haynes |
|
| Northrop et al. (1996) |
|
| Kelley et al. (1975) |
|
| Hilpert (1969) +1 other reference |
|
| Northrop et al. (1996) |
|
| Ferguson (1927) |
|
| Jerry Cone Collection |
|
| USGS Bull 1451 |
|
| USGS Bull 1451 |
- Zuni Mountains Mining District
| Lindgren et al. (1910) |
- Sangre de Cristo Mountains
- Elizabethtown Mining District
| NMGS 38th Field Conference +1 other reference |
|
| Glover et al. (1975) |
|
| Michael C. Michayluk |
|
| NMBMMR Memoir 34 Fluorspar in New Mexico |
|
| Dunham (1935) +1 other reference |
|
| Dunham (1935) +1 other reference |
|
| Northrop et al. (1996) |
|
| McLemore et al. (1996) |
|
| Northrop et al. (1996) |
|
| Virginia T. McLemore (2006) |
- Black Hawk Mining District
| [Rocks & Minerals Vol 68 March/April ... |
- Burro Mountains Mining District
| Gillerman (1964) |
- Deadman Canyon-California Gulch-Whitewater Canyon Area
- Beasley Property (Knucky and Cosgrove)
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| Gillerman (1964) |
- Southwest and Central Burro Mts Area
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| Economic Geology 2:464-492 +1 other reference |
|
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| NMGS 21st Field Conference +1 other reference |
- Carpenter Mining District
| Walstrom (n.d.) |
|
| Northrop et al. (1996) |
|
| Walstrom (n.d.) |
|
| - (1936) |
|
| - (1936) |
|
| - (1936) |
|
| Northrop et al. (1996) |
|
| Lasky (1947) |
|
| Northrop et al. (1996) |
|
| Timothy Hanson +1 other reference |
|
| Northrop et al. (1996) |
|
| - (1991) |
|
| 4th Annual New Mexico Mineral Symposium (1985) |
- U. V. Industries Continental pit
| - (1991) |
|
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| Gillerman (1964) |
|
| Northrop et al. (1996) |
|
| USGS Bull 470 |
|
| Northrop et al. (1996) |
|
| Am Min 38:191-196 +1 other reference |
- Steeple Rock Mining District
| 2008 New Mexico Mineral Symposium ... |
|
| Gillerman (1964) |
|
| collected by Eckhard D. Stuart ... |
|
| 2008 New Mexico Mineral Symposium ... |
|
| 2008 New Mexico Mineral Symposium ... |
- White Signal Mining District
| Gillerman (1964) |
|
| Northrop et al. (1996) |
|
| Clint L. Sandusky and William H. Kaufman (1972) |
|
| Northrop et al. (1996) |
|
| Walstrom (n.d.) |
|
| Walstrom (n.d.) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
- San Simon Mining District
| Robert E.Walstrom Collection |
|
| Northrop et al. (1996) |
|
| Walstrom (n.d.) |
|
| Lindgren et al. (1910) |
- Lordsburg Mining District
| Frank Karasti |
|
| Luetcke (n.d.) |
|
| Walstrom (n.d.) +1 other reference |
|
| Copper Handbook 1911 |
|
| DeMark (1989) |
|
| Walstrom (n.d.) |
|
| Frank Karasti |
|
| Walstrom (n.d.) |
|
| Walstrom (n.d.) |
|
| Walstrom (n.d.) |
|
| Walstrom (n.d.) |
|
| 2009 New Mexico Mineral Symposium ... +1 other reference |
|
| Walstrom (n.d.) |
|
| 2009 New Mexico Mineral Symposium ... |
|
| NMBMMR Memoir 34 Fluorspar in New Mexico |
- Sylvanite Mining District
| Lindgren et al. (1910) +1 other reference |
|
| Lasky (1947) |
|
| Lasky (1947) |
|
| Northrop et al. (1996) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
- Jicarilla Mining District
| Lindgren et al. (1910) |
|
| NMBMGMR Open-file Report - 532 |
|
| Northrop et al. (1996) |
|
| NMBMMR OFR 183 +1 other reference |
|
| Northrop et al. (1996) |
|
| NMBMGMR Open-file Report - 532 |
|
| Anthony et al. (2000) |
|
| NMBMGMR Open-file Report - 532 |
|
| www.newspapers.com (n.d.) |
- Carrizalillo Mining District
| NMBGMR Open-file Report OF-459 |
|
| NMBGMR Open-file Report OF-459 |
- Florida Mountains Mining District
| Griswold (1961) |
|
| Lindgren et al. (1910) |
|
| NMBGMR Open-file Report OF-459 |
|
| Walstrom (n.d.) |
- Tres Hermanas Mining District
| Griswold (1961) |
|
| Jerry Cone Collection |
- Middle Peak (Middle Sisters Peak)
| Griswold (1961) |
- Victorio District (Gage District)
| NMBGMR Open-file Report OF-459 |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Sharpe (1955) |
|
| Lindgren et al. (1910) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Bingler (1968) |
|
| Bingler (1968) |
|
| Lindgren et al. (1910) +1 other reference |
|
| Bingler (1968) |
|
| Lindgren et al. (1910) |
|
| Lindgren et al. (1910) |
|
| Northrop et al. (1996) |
|
| Bingler (1968) +1 other reference |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Lindgren et al. (1910) |
|
| Northrop et al. (1996) |
|
| Lindgren et al. (1910) |
|
| Northrop et al. (1996) |
|
| Lindgren et al. (1910) |
|
| Lindgren et al. (1910) |
|
| Lindgren et al. (1910) +1 other reference |
|
| Bull 167 University of New Mexico +1 other reference |
|
| Lindgren et al. (1910) |
|
| M Massis collection +1 other reference |
|
| Personal communication Ray deMark (2006) |
|
| Elston (1968) |
|
| Lindgren et al. (1910) |
|
| Elston (1968) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| R & J DeMark Minerals of the Carnahan ... |
|
| R&M 67:6 p390-395 +1 other reference |
|
| Maynard (2014) |
- Chloride District (Apache District; Phillipsburg District; Black Range District)
| Harley (1934) |
- Caballo Mountains Mining District
| Harley (1934) |
|
| Harley (1934) |
|
| Harley (1934) |
|
| NMBMMR Memoir 49 Geology of the Caballo ... |
|
| Harley (1934) |
|
| Harley (1934) |
|
| Harley (1934) +1 other reference |
- Hillsboro Mining District
| Harley (1934) |
|
| Harley (1934) |
|
| Visually identified by Patrick Haynes. |
|
| Jerry Cone Collection |
|
| Jerry Cone Collection |
|
| Harley (1934) |
|
| Northrop et al. (1996) |
- Mockingbird Gap Mining District
| Lasky (1932) |
- Pittsburg Mining District
| Harley (1934) |
- Salinas Peak Mining District
| Lasky (1932) |
- Sierra Cuchillo (Cuchillo Negro Mts)
| Northrop et al. (1996) |
|
| Harley (1934) |
|
| Northrop et al. (1996) |
|
| Harley (1934) |
|
| Northrop et al. (1996) |
|
| Patrick Haynes |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| [Mineralogical Record 30:337] |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| McLemore et al. (2009) |
|
| Gibbs (1989) |
|
| GF Loughlin (1942) |
|
| M Massis collection |
|
| Rex Nelson |
|
| GF Loughlin (1942) |
|
| M Massis collection |
|
| Patrick Haynes |
|
| GF Loughlin (1942) |
|
| M Massis collection |
|
| Ex. New Mexico Bereau of Mines and ... |
|
| GF Loughlin (1942) |
|
| Northrop et al. (1996) |
- North Magdalena Mining District
| Rockhounding New Mexico +2 other references |
- Ojo Caliente No. 2 Mining District
| NMBGMR Open-file Report - 535 |
|
| Northrop et al. (1996) |
- San Lorenzo Mining District
| Page et al. (1956) +2 other references |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Northrop et al. (1996) |
|
| Schilling (1960) |
|
| Northrop et al. (1996) |
|
| Jahns et al. (1977) |
|
| Schilling (1956) +1 other reference |
- Red River Mining District
| Schilling (1960) |
|
| NMBMMR Bull 94 Geology and Ore Deposits ... |
|
| NMBMMR Bull 94 Geology and Ore Deposits ... +2 other references |
|
| Northrop et al. (1996) |
|
| NMBMMR Bull 94 Geology and Ore Deposits ... +2 other references |
|
| NMBMMR Bull 94 Geology and Ore Deposits ... +2 other references |
|
| NMBMMR Bull 94 Geology and Ore Deposits ... |
|
| Schilling (1960) |
|
| Richard Dale Collection |
|
| Northrop et al. (1996) |
|
| NMGS 38th Field Conference |
|
| Northrop et al. (1996) |
|
| NMGS 38th Field Conference |
|
| Fulp et al. (1982) |
- Manhattan (New York County)
| John H. Betts (2009) |
|
| Jensen (1978) |
|
| Observed/collected-David Bernstein-Jan. ... |
|
| Goble et al. (1980) |
|
| Econ Geol (1990) +1 other reference |
|
| F. Rutley: "Elements of Mineralogy" (1900) |
|
| Whitlock (1903) |
|
| Manchester (1931) |
|
| Wilson et al. (1978) |
|
| Rocks & Min.: 60:86. |
|
| |
|
| Wilson et al. (1978) |
|
| Dana 6:1075. |
|
| Dana 6:1075. |
|
| Genth +1 other reference |
- Chatham County Copper Belt
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| J.Wright Horton |
- Silver Hill Mining District
| Most blue spots and especially crystals are linarite. However (affirming carbonate composition) |
|
| Genth +1 other reference |
|
| NCGS Bulletion 26 +1 other reference |
|
| Rocks & min.:60:90. |
|
| Rocks & min.:60:90. |
|
| |
|
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| Mineral Commodity Files |
|
| Rocks & Min.:60:91. |
|
| Genth (1891) +1 other reference |
|
| Carpenter III (1976) |
|
| Rocks & Min.:60:92. |
|
| Dana 6:1078. |
|
| Carpenter III (1976) |
|
| |
|
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| |
|
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| U.S Bureau of Mines/Spatial Data from the Minerals System/Mineral Industry Location System (Mas/Mils) |
|
| Carpenter III (1976) |
|
| Bryson et al. (1928) |
|
| Carpenter III (1976) |
|
| |
|
| Wilson et al. (1978) |
|
| Rocks & Min. vol. 72 (1997) |
|
| R&M 72:4 pp 252-264 |
|
| R&M 72:4 pp 252-264 |
|
| Rocks & Min. vol. 72 (1997) |
|
| - (2005) |
|
| - (2005) |
|
| R&M 72:4 pp 252-264 |
|
| Lobell (1986) |
|
| Rocks & Min. vol. 72 (1997) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| R&M 72:4 pp 252-264 |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| - (2005) |
|
| R&M 72:4 pp 252-264 |
|
| Fay (1981) |
|
| R&M 72:4 pp 252-264 |
|
| R&M 72:4 pp 252-264 |
|
| Lisa19 |
|
| Rocks & Min. vol. 72 (1997) |
|
| - (2005) |
- Butte Creek Mining District
| Oregon Metal Mines Handbook |
- Evans Creek Mining District
| Oregon Metal Mines Handbook |
|
| Oregon Metal Mines Handbook |
|
| Oregon Metal Mines Handbook |
|
| Oregon Metal Mines Handbook (1952) |
|
| Oregon Metal Mines Handbook (1952) |
|
| |
|
| - (prospector field notes) |
|
| Gordon |
|
| |
|
| Gordon |
|
| Gordon |
|
| Gordon |
|
| Gordon |
|
| Gordon |
|
| Gordon |
|
| |
|
| Ref.: Gordon (1922) |
|
| Gems and Minerals of America- Jay Ellis ... |
|
| The Mineralogy of Pennsylvania |
|
| Sloto (1989) |
|
| Rocks & Min.:9:21. |
|
| Smith (1855) +1 other reference |
|
| Mineralogical Record (2) |
|
| The Mineralogy of Pennsylvania 1966-1975 +1 other reference |
- Cornwall Borough (Cornwall)
| Lapham & Geyer |
|
| Smith et al. (1984) |
|
| various added photos |
|
| Smith and Hoff (1984) |
|
| Smith (1977) |
|
| Smith and Hioff (1984) |
|
| Gems and Minerals of America- Jay Ellis ... |
- Lower Providence Township
| Rocks & Min.: 21:17. +2 other references |
|
| Boucot (1949) |
|
| Smith (1977) |
|
| A. Geyer et al. (1976) |
- C.K. Williams Quarry complex
| Rocks & Min.: 17:344. |
|
| Gems and Minerals of America- Jay Ellis ... |
|
| www.pennminerals.com (n.d.) |
|
| Lapham & Geyer |
|
| Broedel (1961) |
|
| Miller |
|
| Miller (1971) |
|
| AmMin 22:279 +2 other references |
|
| |
|
| Rocks & Min.: 20:463-464. +1 other reference |
|
| Michael W. Kieron collection |
|
| Rocks & Minerals: 60: 117-118. |
|
| Roberts et al. (1965) |
|
| Smith et al. (2000) |
|
| Smith et al. (2000) |
|
| Roberts et al. (1965) |
|
| Loomis (1999) |
|
| Roberts et al. (1965) |
- Hill City Mining District
| Overman (1935) |
|
| Roberts et al. (1965) |
|
| Rocks & Minerals: 67 (6) |
|
| Am Min 70:9-10 pp 1044-1049 |
|
| |
|
| Roberts et al. (1965) |
|
| Roberts et al. (1965) |
|
| Paris (2011) |
|
| www.gamineral.org +1 other reference |
|
| Richard (1915) |
|
| Richard. LM (1915) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Rocks & Minerals: 66 (3) |
- Van Horn-Allamoore Mining District
| Smith (1991) |
|
| Smith (1991) |
- Franklin Mountains (Franklin Range)
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| BEG Min Res Circ 73 |
|
| BEG Min Res Circ 73 |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| BEG Min Res Circ 73 |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Price et al. (1985) |
|
| BEG Min Res Circ 73 |
|
| Smith (1991) |
|
| Smith (1991) |
|
| BEG Min Res Circ 73 |
- Van Horn-Allamoore Mining District
| BEG Min Res Circ 73 |
|
| BEG Min Res Circ 73 |
|
| BEG Min Res Circ 73 |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Smith (1991) |
|
| - (2005) |
|
| Min News 9:10 p2 |
|
| Smith (1991) |
|
| BEG Min Res Circ 73 |
|
| BEG Min Res Circ 73 |
|
| Smith (1991) |
|
| Smith (1991) |
|
| Rocks & Min. |
|
| Barton et al. (2018) |
- Beaver Lake Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Butler (1913) +1 other reference |
|
| Butler (1913) +1 other reference |
|
| Butler (1913) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- San Francisco Mining District
| Butler (1913) +1 other reference |
- Preuss Mining District (Newhouse Mining District)
| Bullock (1981) |
|
| Bullock (1981) |
- Star-North Star Mining District
| Butler (1913) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Butler (1913) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Butler (1913) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Butler (1913) +1 other reference |
|
| Butler (1913) +1 other reference |
|
| Bullock (1981) |
|
| Butler (1913) +1 other reference |
|
| R&M 66:1 p24-28 |
|
| Bullock (1981) |
- Grouse Creek Mts (Grouse Creek Range)
| Bullock (1981) |
|
| Bullock (1981) |
- Newfoundland Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Utah Geological Survey (2018) |
|
| USGS: Geological Survey Circular 312 +1 other reference |
|
| Bullock (1981) |
- Farmington Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Econ Geol (1996) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- San Rafael Swell Mining District
| Bullock (1981) |
|
| - (2005) |
|
| Page et al. (1956) +3 other references |
|
| Page et al. (1956) +2 other references |
- Circle Cliffs Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Henry Mountains Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- La Sal Mountain Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Seven Mile Canyon Mining District
| Luetcke (n.d.) |
- Thompsons Mining District
| Bullock (1981) |
- Iron Springs Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Pinto Iron Mining District (Pinto Mining District; Silver Belt Mining District)
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Spring Creek Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Rick Dalrymple Collection |
|
| Bullock (1981) |
|
| Roberts et al. (1997) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| R&M 66:1 p24-28 |
|
| Bullock (1981) |
|
| Thorne (n.d.) |
|
| Bullock (1981) |
|
| Dana 6: 1092 +2 other references |
|
| Robert O. Meyer collection +1 other reference |
|
| Collected by and in the collection of ... |
|
| Thorne (n.d.) |
|
| R&M 66:1 p14-23 |
- Victor Mine (Red Rose Mine; Boss Tweed Mine)
| Bullock (1981) |
|
| Bullock (1981) |
- West Tintic Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Detroit Mining District (Drum Mining District; Joy Mining District)
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Leamington Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Gold Mountain Mining District (Kimberly Mining District)
| Bullock (1981) |
- Mount Baldy-Ohio Mining District
| Bullock (1981) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Swan Creek District (Garden City District)
| USGS Bull 470 +1 other reference |
- Big Cottonwood Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Laurence P. James (1979) |
|
| Calkins +4 other references |
|
| UGMS B-114 (James,1979) |
|
| Calkins +3 other references |
|
| Rocks & Min.: 10:111-112. +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Little Cottonwood Mining District
| Bullock (1981) |
|
| U.S. Geological Survey Professional ... +1 other reference |
- Abajo Mountains Mining District
| U.S. Geological Survey Professional ... +1 other reference |
|
| Finnell et al. (1963) |
|
| USGS Bull 1125 |
|
| Finnell et al. (1963) +1 other reference |
- Elk Ridge Mining District
| Bullock (1981) |
|
| Bullock (1981) |
- Fry Canyon Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- La Sal Creek Mining District
| Carter et al. (1965) +1 other reference |
|
| Bullock (1981) |
|
| |
- Lisbon Valley Mining District
| Econ Geol (1986) |
|
| Min News 10:12 p5 +4 other references |
|
| |
|
| various added photos |
|
| Wilson (1990) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Collection of Alex Earl |
|
| Bullock (1981) |
- Red Canyon Mining District
| collrcted by and in the collection of ... |
|
| Bullock (1981) |
|
| Bullock (1981) |
- White Canyon Mining District
| Bullock (1981) |
|
| Dr. Jeffrey Weissman specimen |
|
| USGS TEI #514 +4 other references |
|
| Trites et al. (1958) +2 other references |
- Salina Canyon Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Park City Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Dugway Mountains Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Rick Dalrymple Collection |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Granite Mountain Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Gold Hill Mining District (Clifton Mining District)
| Nolan et al. (1935) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Collected by and in the collection of ... |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Kokinos et al. (1993) |
|
| Kokinos et al. (1993) |
|
| Thorne (n.d.) |
|
| Mineralogical Record: 24 (1) +1 other reference |
|
| Thorne (n.d.) +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Pole Star Copper Company Mine Group
| Bullock (1981) |
|
| Bullock (1981) |
|
| Thorne (n.d.) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Schempp et al. (1931) +17 other references |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Rocks & Min.: 20: 213. |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Rocks & Minerals 83:1 pp 52-62 |
|
| Bullock (1981) |
|
| Thorne (n.d.) +1 other reference |
|
| R&M 66:1 p24-28 |
- East Erickson Mining District
| Bullock (1981) |
- Silver Island Mining District
| Bullock (1981) |
- Third Term Mining District
| USGS MRDS Database |
|
| Buranek (1945) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Brush Creek Mining District
| Bullock (1981) |
- Carbonate Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- American Fork Mining District
- Miller Hill Mine Group (Miller Hill Mining Co. claims)
| Calkins +3 other references |
|
| - (2005) |
|
| Bullock (1981) |
- East Tintic Mining District
| U.S. GEOLOGICAL SURVEY PROFESSIONAL ... +1 other reference |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Waldemar Lindgren and G. F. Loughlin with a historical review by V. Conrad Heikes (1919) |
|
| USGS Prof Paper 1024 +1 other reference |
|
| Lindgren |
|
| U.S. GEOLOGICAL SURVEY PROFESSIONAL ... +1 other reference |
- Tutsagubet Mining District
| MacFall (1951) +1 other reference |
- Mineral Mountain Mining District
| Bullock (1981) |
|
| Carl L. Ege (2005) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
- Miners Mountain Mining District
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Bullock (1981) |
|
| Rocks & Min.: 22: 842 +1 other reference |
|
| Nicolay & Stone (1967) |
|
| |
|
| Bulletin 77. +2 other references |
|
| Dietrich (1990) |
|
| Rocks & Min.:60:165. |
|
| The Northern Virginia Mineral Club (2009) |
- Southern Section-Blue Ridge Province
- Gossan Lead Mining District
| Dietrich (1990) |
- Virgilina Mining District
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Rocks & Min.: 60:166. |
|
| Dietrich (1990) |
- Northern Section-Blue Ridge Province
| Dietrich (1990) |
- Virgilina Mining District
| Copper Handbook 1911 |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Rocks & Min.: 60:167. |
|
| Dietrich (1990) |
|
| Brander Robinson Collection |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
- Northern Section-Blue Ridge Province
| Dietrich (1990) |
|
| Rocks & Min.:60:167. +1 other reference |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
- Northern Section-Blue Ridge Province
| Rocks & Min.:60:168. +2 other references |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| D. M. Schlegal (1959) |
|
| Dietrich (1990) |
|
| D. M. Schlegal (1959) |
- Northern Section-Blue Ridge Province
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
|
| Dietrich (1990) |
- Blewett Mining District (Peshastin Mining District)
| John Lindell Collection |
- Covada Mining District (Enterprise Mining District)
| Derkey et al. (1990, November) +1 other reference |
|
| Valentine et al. (1960) +2 other references |
|
| R. Derkey +3 other references |
- San Poil Mining District (Keller Mining District)
| R. Derkey +6 other references |
|
| Copper Handbook 1911 |
|
| Valentine et al. (1960) +1 other reference |
- Buena Vista Mining District
| Singer et al. (2008) |
- Snoqualmie Mining District
- Middle Fork of the Snoqualmie River
| Singer et al. (2005) |
- North Fork of the Skokomish River Area
| R. Derkey +3 other references |
- Conconully Mining District
| Derkey et al. (1990, November) |
|
| Singer et al. (2008) |
|
| Valentine et al. (1960) +1 other reference |
- Myer Creek Mining District
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
- Nighthawk Mining District
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Valentine et al. (1960) +2 other references |
|
| R. Derkey +1 other reference |
|
| Valentine et al. (1960) +1 other reference |
- Palmer Mountain Mining District
| R. Derkey +3 other references |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
- Park City Mining District
| Valentine et al. (1960) +1 other reference |
|
| Valentine et al. (1960) +2 other references |
|
| Cannon (1975) |
|
| Bart Cannon analysis |
|
| Cannon (1975) |
|
| USGS Bull 470 +1 other reference |
|
| Cannon (1975) |
|
| Cannon (1975) |
|
| Singer et al. (2008) |
- Washougal Mining District
| Moen (1977) |
|
| Valentine et al. (1960) +1 other reference |
|
| Moen et al. (1977) |
- Glacier Peak Mining District
| Derkey et al. (1990, November) |
|
| Derkey et al. (1990, November) +1 other reference |
- Silver Creek Mining District
| Jacobson et al. (2008) |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Cannon (1975) |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Cannon (1975) |
|
| Cannon (1975) |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
|
| Cannon (1975) |
- Clugston Creek Mining District
| R. Derkey +3 other references |
- Deer Trail Mining District
| Moen (1964) |
|
| Valentine et al. (1960) |
|
| Valentine et al. (1960) +1 other reference |
|
| R. Derkey +3 other references |
|
| Cannon (1975) |
- Kettle Falls Mining District
| Derkey et al. (1990, November) +1 other reference |
- Northport Mining District
| Derkey et al. (1990, November) +1 other reference |
|
| Derkey et al. (1990, November) +1 other reference |
- Springdale Mining District
| Derkey et al. (1990, November) +1 other reference |
|
| Cannon (1975) +2 other references |
- Mount Baker Mining District
| Cannon (1975) |
|
| Moen (1969) |
- Bumping Lake Mining District
| Valentine et al. (1960) |
|
| Cordua +1 other reference |
|
| R&M 73:11-12 pp 378-399 Wisconsin ... |
|
| Cordua |
- Upper Mississippi Valley Mining District
| Heyl et al. (1959) |
|
| Heyl et al. (1959) |
- Upper Mississippi Valley Mining District
| Heyl et al. (1959) |
|
| Heyl et al. (1959) |
|
| Cordua (1998) |
- Mineral Point copper deposits
| Heyl et al. (1959) |
|
| Heyl et al. (1959) +1 other reference |
|
| Dana 6:297. |
|
| Heyl et al. (1959) |
|
| Heyl et al. (1959) |
|
| Rocks & Min. Vol. 73 (1998) |
|
| Cordua (1998) |
|
| Heyl et al. (1959) |
|
| Cordua (1998) |
- Ladysmith-Rhinelander Metavolcanic Complex
| Jones +2 other references |
|
| Cordua (1998) |
|
| Rocks & Minerals 76:382 |
|
| Rocks & Minerals 76:382 |
|
| Rocks & Minerals 76:382 |
|
| Wyoming State Geological Survey +2 other references |
|
| Rocks & Minerals 76:383 |
- Big Creek pegmatite District
| Frank Karasti |
|
| Rocks & Minerals 76:384 |
|
| Dake (1956) |
|
| - (2005) |
|
| Spencer (1904) |
|
| Spencer (1904) +1 other reference |
|
| - (2005) |
|
| Dake (1956) |
|
| Spencer (1904) |
|
| - (2005) |
|
| - (2005) |
|
| Rocks & Minerals 76:385 |
|
| Jay Ellis Ransom |
|
| Rocks & Minerals 76:386 |
|
| USGS Bull 315 |
|
| Rocks & Minerals (2001) |
|
| USGS Bull 315 |
|
| - (2005) +1 other reference |
|
| Rocks & Minerals 76:388 |
|
| Hausel (2012) |
|
| Wyoming Geological Survey |
|
| Rocks & Minerals 76:388 +2 other references |
|
| Rocks & Minerals 76:388 |
|
| Singer et al. (2008) |
|
| Rocks & Minerals 76:388 |
|
| Rocks & Minerals 76:389 |
|
| USGS Bull 315 |
|
| R&M 69:3 pp 163-168 +1 other reference |
|
Uzbekistan | |
|
| Eilu (2003) +1 other reference |
|
| Eilu (2003) |
|
Venezuela | |
|
| Urbani (1996) |
|
| Franco Urbani (2009) |
|
Vietnam | |
| |
|
| Jadwiga PIECZONKA et al. (2019) |
|
Zambia | |
|
| Korowski et al. (1980) |
|
| Hendrickson (2013) |
|
| - (2005) +1 other reference |
|
| www.portergeo.com.au (n.d.) |
|
| |
|
| Info-Mine Property Summary "Mufulira ... |
|
| |
|
| Jacobs (2016) |
|
Zimbabwe | |
|
| WILSON et al. (1998) |
|
| Cairncross (2004) |
|
| Cairncross (2004) |
|
| Cairncross (2004) |
|
| Cairncross (2004) +1 other reference |
|
| Cairncross (2004) |
|
| Cairncross (2004) |
- Chiredzi mining district (Hartley mining district)
| Bartholomew (1990) +1 other reference |
- Umkondo mining area (Mkondo)
| Cairncross (2004) |
|
| Bahnemann |
|
| Cairncross (2004) |
|
| Vetter et al. (1999) +1 other reference |
|
| Cairncross (2004) |
 Visit gemdat.org for gemological information about Azurite.
Visit gemdat.org for gemological information about Azurite. Visit gemdat.org for gemological information about Azurite.
Visit gemdat.org for gemological information about Azurite.
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).



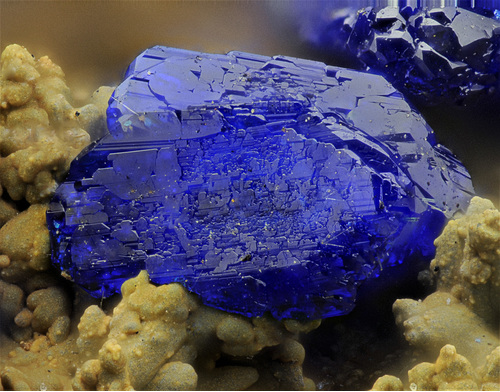




 symbol to view information about a locality.
The
symbol to view information about a locality.
The 





Lavrion Mining District, Lavreotiki, East Attica, Attica, Greece